Abstract
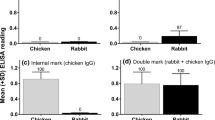
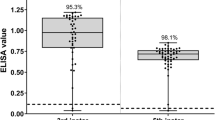
We introduce a new method for immunologically examining predator gut contents. It differs from previously described gut content analyses because it does not require the development of prey-specific antibody probes. Instead, insect prey were marked with a readily available antigen, rabbit immunoglobulin G (IgG). We then assayed predators that had fed on IgG labeled prey with an enzyme-linked immunosorbent assay (ELISA) using goat anti-rabbit IgG. Of the predator species that fed on the IgG labeled prey, 98.8% of those with chewing mouthparts scored positive for IgG 1 h after feeding. Our prey-labeling ELISA was less efficient for detecting IgG prey remains in predators with piercing/sucking mouthparts. Only 29.5% of these individuals scored positive for rabbit IgG in their guts 1 h after feeding. An additional study was conducted to measure the retention time of IgG-labeled prey in the guts of two species of predators with chewing mouthparts. Results from this experiment showed that the retention time varied depending on the predator and prey species examined. Results from these studies indicate that this marking technique could have widespread use for analyzing the gut contents of predators with chewing mouthparts, but it has limited application for those predators with piercing/sucking mouthparts.
Résumé
La nouvelle méthode d'analyse immunologique du contenu du tube digestif des insectes prédateurs diffère des méthodes précédemment décrites car elle ne demande pas le développement préalable d'anticorps spécifiques de la proie. Au contraire la proie est marquée avec un antigène de mammifère déjà disponible, l'immunoglobuline G de lapin (IgG). Nous avons donc testé cette méthode sur des prédateurs nourris de proies marquées par l'immunoglobuline par la technique ELISA en utilisant un anticorps de l'immunoglobuline de lapin. Parmi les espèces de prédateurs nourries de proies marquées à l'immunoglobuline, 98,8% d'entre elles (du type broyeur) ont réagi positivement à l'immunoglobuline 1 heure après s'être nourries. Notre technique de marquage de proie ELISA est moins efficace pour détecter les restes de proies marqués à l'immunoglobuline chez les prédateurs de type suceur-piqueur. Seulement 29,5% de ces derniers présentent une réaction positive à l'immunolobuline dans leur tube digestif lh après s'être nourris. Les résultats de cette étude montrent que cette technique de marquage pourrait être largement utilisée pour analyser le contenu intestinal des prédateurs de type broyeur mais qu'elle présente un intérêt limité pour les prédateurs de type suceur-piqueur.
Similar content being viewed by others
References
Baldwin, W. F., James, H. G. &Welch, H. E. — 1955. A study of predators of mosquito larvae and pupae with a radio-active tracer. —Can. Entomol. 87, 350–356.
Booij, C. J. H. &Noorlander, J. — 1992. Farming systems and insect predators. —Agric. Ecosystems Environ., 40, 125–135.
Breene, R. G. &Sterling, W. L. — 1988. Quantitative phosphorus-32 labeling method for analysis of predators of the cotton fleahopper. —J. Econ. Entomol., 81, 1494–1498.
Byrne, D. N. &Hadley, N. F. — 1988. Particulate surface waxes of whiteflies: morphology, composition and waxing behavior. —Physiol. Entomol., 13, 267–276.
Hagler, J. R. &Cohen, A. C. — 1990. Effects of time and temperature on digestion of purified antigen byGeocoris punctipes reared on artificial diet. —Ann. Entomol. Soc. Am., 83, 1177–1180.
Hagler, J. R., Cohen, A. C., Enriquez, F. J. &Bradley-Dunlop, D. — 1991. An egg specific monoclonal antibody toLygus hesperus. —Biological Control, 1, 75–80.
Hagler, J. R., Cohen, A. C., Bradley-Dunlop, D. &Enriquez, F. J. — 1992a. Field examination of predation onLygus hesperus using a species-and stage-specific monoclonal antibody. —Environ. Entomol., 21, 896–900.
Hagler, J. R., Cohen, A. C., Bradley-Dunlop, D. &Enriquez, F. J. — 1992b. New approach mark insects for feeding and dispersal studies. —Environ. Entomol., 21, 20–25.
Hagler, J. R. &Naranjo, S. E. — 1994a. A qualitative survey of two coleopteran predators ofBemisia tabaci andPectinophora gossypiella using a multiple prey gut content ELISA. —Environ. Entomol., 23: 193–197.
Hagler, J. R. &Naranjo, S. E. — 1994b. Determining the frequency of heteropteran predation on sweetpotato whitefly and pink bollworm using multiple ELISAs. —Entomol. Exp. Appl., 72: 59–66.
Hengeveld, R. — 1980. Qualitative and quantitative aspects of the food of ground beetles. A review. —Netherlands J. Zool., 30, 555–563.
Henneberry, T. J. & Clayton, T. E. — 1985. Consumption of pink bollworm and tobacco budworm eggs by some predators commonly found in cotton fields. —Environ. Entomol., 416–419.
Hsu, H. T., Vongasaitorn, D. &Lawson, R. H. — 1992. An improved method for serological detection of cymbidium mosaic potexvirus infection in orchids. —Phytopathology, 82, 491–495.
James, H. G. — 1961. Some predators ofAedes stimulans andAedes trichurus in woodland pools. —Can. J. Zool., 39, 533–540.
Lenz, C. J. &Greenstone, M. H. — 1988. Production of a monoclonal antibody to the arylphorin ofHeliothis zea. —Arch. Insect Biochem. Physiol., 9, 167–177.
Luff, M. L. — 1983. The potential of predators for pest control. —Agric. Ecosystems Environ., 10, 159–181.
McCarty, M. T., Shepard, M. &Turnipseed, S. G. — 1980. Identification of predaceous arthropods in soybeans by using autoradiography. —Environ. Entomol., 9, 199–203.
McDaniel, S. G., Keeley, L. L. &Sterling, W. L. — 1978. RadiolabelingHeliothis virescens eggs by32P injection of adult females. —Ann. Entomol. Soc. Am., 71, 432–434.
McIver, J. D. — 1981. An examination of the utility of the precipitin test for evaluation of arthropod predator-prey relationships. —Can. Entomol., 113, 213–222.
Murray, R. A. &Solomon, M. G. — 1978. A rapid technique for analysing diets of invertebrate predators by electrophoresis. —Ann. Appl. Biol., 90, 7–10.
Orphanides, G. M., Gonzales, D. &Bartlett, B. R. — 1971. Identification and evaluation of pink bollworm predators in southern California. —J. Econ. Entomol., 64, 421–424.
Stuart, M. K. &Greenstone, M. H. — 1990. Beyond ELISA: a rapid, sensitive, specific immunodot assay for identification of predator stomach contents. —Ann. Entomol. Soc. Am., 83, 1101–1107.
Sunderland, K. D. — 1987. A review of methods of quantifying invertebrate predation occurring in the field. —Acta. Phytopath. Entomol. Hung., 22, 13–34.
Sutula, C. L., Gillett, J. M., Morrissey, S. M. &Ramsdell, D. C. — 1986. Interpreting ELISA data and establishing the positive-negative threshold. —Plant Disease, 70, 722–726.
Symondson, W. O. C. &Liddell, J. E. — 1993. A monoclonal antibody for the detection of arionid slug remains in carabid predators. —Biol. Control., 3, 207–214.
Tijssen, P. — 1985. Practice and Theory of Enzyme Immunoassays Vol. 15. Lab Techniques in Biochemistry and Molecular Biology. —Elsevier, Amsterdam, Holland.
Author information
Authors and Affiliations
Additional information
This article presents the results of research only. Mention of a proprietary product does not constitute an endorsement or recommendation for its use by the USDA.
Rights and permissions
About this article
Cite this article
Hagler, J.R., Durand, C.M. A new method for immunologically marking prey and its use in predation studies. Entomophaga 39, 257–265 (1994). https://doi.org/10.1007/BF02373030
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02373030