Conclusions
-
1.
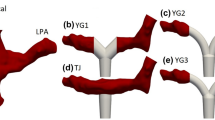
Coaxial and angular shift of anastomosis have significant effects on the flow rate distribution, size and location of recirculation zones, and zones of vortex formation or congestion.
-
2.
To reduce the hemodynamic complications caused by blood flow distortion, the CCPA anastomosis is recommended to be made at an angle of 60±5° to the RPA axis and with a 0.5d±0.1d axial shift.
-
3.
If the anastomosis is shifted by more than 0.5d, a steady state macrovortex is formed at the central area of the CCPA. The vortex is a closed recirculation zone (three-dimensional coil) capable of complete obstruction of the blood vessel lumen.
-
4.
Inadequately applied anastomosis may cause blood flow destructurization, increase the probability of hemolysis, ischemia, and thrombogenesis, and provoke postoperative arrhythmia caused by flow rate pulsations.
Similar content being viewed by others
Literature Cited
E. V. Len'ko, E. E. Litasova, V. V. Pai, et al., Proc. 2nd All-Russian Congr. of Cardiovascular Surgeons, St. Petersburg (1993), pp. 12–13.
V. P. Podzolkov, M. R. Chiaureli, S. B. Zaets, et al., Grudnaya Serd.-Sosud. Khir., No. 6, 11–16 (1990).
H. S. Bossiouny, S. White, S. Glagov, et al., J. Vasc. Surg.,15, 708–716 (1992).
H. M. Crawshaw, W. C. Quist, E. Serralach, et al., Arch. Surg.,15, 1280–1284 (1980).
R. S. Keynton, S. E. Rittgers, and M. C. Shu, J. Biomech. Eng.,113, 458–463 (1991).
J. K. Kirklin, E. H. Blackstone, J. W. Kirklin, et al., J. Thorac. Cardiovasc. Surg.,92, 1049–1064 (1986).
M. R. de Leval, P. Kilner, M. Gewilling, and C. Bull, J. Thorac. Cardiovasc. Surg.,96, 682–692 (1988).
D. D. Mair, V. J. Rice, D. J. Hagler, et al., Circulation,72, No. 2, 88–92 (1985).
E. J. Puga, J. Thorac. Cardiovasc. Surg.,98, 150–154 (1989).
S. E. Rittgers and G. H. Bhambhani, Ultrasound Med. Biol.,19, 257–267 (1993).
V. S. Sottiurai, J. S. T. Yao, W. R. Flinn, and R. C. Baston, Surgery,94, No. 6, 809–817 (1983).
V. S. Sottiurai, S. L. Sue, E. L. Feinberg, et al., Eur. J. Vasc. Surg.,2, 245–256 (1988).
V. S. Sottiurai, J. S. T. Yao, R. C. Baston, et al., Ann. Vasc. Surg.,3, 26–33 (1989).
N. H. Staalsen, M. Ulrich, et al., J. Vasc. Surg.,21, 460–471 (1995).
S. S. White, C. K. Zarins, D. P. Giddens, et al., J. Biomech. Eng.,115, 104–111 (1993).
Additional information
Research Center of Cardiovascular Surgery, Russian Academy of Medical Sciences. Institute of Physics and Technology, Moscow. Translated from Meditsinskaya Tekhnika, No. 5, pp. 18–24, September–October, 1996.
Rights and permissions
About this article
Cite this article
Roeva, L.A., Meshkov, M.A. & Chubarova, E.Y. Comparative hydrodynamic evaluation of variants of cavapulmonary anastomosis. Biomed Eng 30, 260–267 (1996). https://doi.org/10.1007/BF02369077
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02369077