Abstract
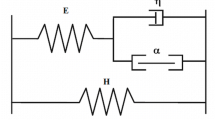
A dynamic nonlinear lumped parameter model of the circulation of skeletal muscle for constant vasoactive state is presented. This model consists of four compartments that represent the large arteries, the arterioles, the capillaries and venules, and the veins, respectively. The first compartment consists of a linear compliance (C1) and resistance (R1). The third compartment possesses no compliance and is represented by a linear resistance (R3). The second and fourth compartments each consist of a nonlinear pressure-volume relation, resulting in a pressure dependent compliance (C2, C4, respectively) and nonlinear resistance (R2, R4, respectively). The eleven model parameters were collected in a complementary way: they were partly obtained from a priori knowledge including, information at the microscopic level, and partly determined by means of an estimation algorithm. Estimated values of the compliances (in cm3·kPa−1·100 g−1, 1kPa=7.5 mmHg) and resistances (in kPa·s·cm−3·100 g) at an (arterial) inflow pressure of 10 kPa and a (venous) outflow pressure of 0 kPa were: C1: 0.014; R1: 6.6; C2: 0.565; R2: 84.6; R3: 37.9; C4: 1.044; R4: 24.5. The model (with the nonlinear pressure-volume relations) is able to predict the static and dynamic instantaneous (i.e., for constant vasomotor tone) pressure-flow relation and the instantaneous zero flow pressure intercept. These phenomena are therefore not necessarily the result of the rheological properties of blood. The secondary or delayed dilatation upon a positive inflow pressure step (or negative step in venous pressure) is predicted by the model implying that delayed dilatation is not necessarily related to changes in vasomotor tone. Venous outflow delay, upon a positive inflow pressure step (starting from zero flow), is also predicted by the model.
Similar content being viewed by others
Abbreviations
- A :
-
slope ofP-V relation atV 0
- C :
-
compliance (dV/dP)
- D :
-
diameter
- F :
-
flow
- F in :
-
input (arterial) flow, model
- R out :
-
output (venous) flow, model
- F p :
-
input (arterial) flow, measurements
- F v :
-
output (venous) flow, measurements
- i :
-
generation number
- K :
-
8νπL 3
- K eff :
-
effective value ofK
- L :
-
length
- N :
-
number of vessels
- P :
-
pressure
- P in :
-
input (arterial) pressure, model
- P out :
-
output (venous) pressure, model
- P 0a :
-
apparent zero flow pressure intercept
- P 0i :
-
instantaneous zero flow pressure intercept
- P 0s :
-
steady state zero flow pressure intercept
- P p :
-
perfusion (arterial) pressure, measurements
- P v :
-
output (venous) pressure, measurements
- R :
-
resistance
- t :
-
time
- V :
-
volume
- V 0 :
-
half maximal volume of compartment
- V 0ef :
-
half effective maximal volume of compartment
- W :
-
working point
- ν:
-
viscosity
- τ:
-
time constant
References
Baez, S. Response characteristics of perfused microvessels. Angiology 12:452–461; 1961.
Baker, C.H.; Davis, D.L. Isolated skeletal muscle blood flow and volume changes during contractile activity. Blood Vessels 11:32–44; 1974.
Baker, C.H.; Menninger, R.P.; Schoen, R.E.; Truitt Sutton, E. Skeletal muscle vascular volume changes with increased venous pressure. Blood Vessels 13:222–237; 1976.
Best, M.; Blumenthal, M.; Masket, S.; Galin, M.A. Effects of sympathetic stimulation on critical closure of intraocular blood vessels. Invest. Ophthalmol. 9:911–916; 1970.
Braakman, R.; Sipkema, P.; Westerhof, N.. Steady state and instantaneous pressure-flow relationships: characterization of the canine iliac periphery. Cardiovasc. Res. 17:577–588; 1983.
Braakman, R. Pressure-flow relationships in skeletal muscle. From measurement to model. Amsterdam: Free University; 1988. Dissertation.
Bruinsma, P.; Arts, T.; Dankelman, J.; Spaan, J.A.E. Model of the coronary circulation based on pressure dependence of coronary resistance and compliance. Bas. Res. in Cardiol. 83:510–524; 1988.
Cox, R.H. Arterial wall mechanics and composition and the effects of smooth. Am. J. Physiol. 229: 807–812; 1975.
Duling, B.R.; Sarelius, I.G.; Jackson, W.F. A comparison of microvascular estimates of capillary blood flow with direct measurements of total striated muscle flow. Int. J. Microcirc. Clin. Exp. 1: 409–424; 1982.
Ehrlich, W.; Baer, R.W.; Bellamy, R.F.; Randazzo, R. Instantaneous femoral artery pressure-flow relations in supine anesthetized dogs and the effect of unilateral elevation of femoral venous pressure. Circ. Res. 47:88–98; 1980.
Eng, C.; Kirk, E.S. Flow into ischemic myocardium and across coronary collateral vessels is modulated by a waterfall mechanism. Circ. Res. 55:10–17; 1984.
Engelson, E.T.; Schmid-Schönbein, G.W.; Zweifach, B.W. The microvasculature in skeletal muscle. III Venous network anatomy in normotensive and spontaneously hypertensive rats. Int. J. Microcirc. 4:229–248; 1985a.
Engelson, E.T.; Skalak, T.C.; Schmid-Schönbein, G.W. The microvasculature in skeletal muscle. I. Arteriolar network in rat spinotrapezius muscle. Microvasc. Res. 30:29–44; 1985b.
Engelson, E.T.; Schmid-Schönbein, G.W.; Zweifach, B.W. The microvasculature in skeletal muscle II. Arteriolar network anatomy in normotensive and spontaneously hypertensive rats. Microvasc. Res. 31:356–374; 1986.
Eriksson, E.; Myrhage, R. Microvascular dimensions and blood flow in skeletal muscle. Acta Physiol. Scand. 86:211–222; 1972.
Ganong, W.F. Review of medical physiology. Los Altos, CA: Lange Medical Publications; 1985.
Greensmith, J.E.; Duling, B.R. Morphology of the constricted arteriolar wall: physiological implications. Am. J. Physiol. 247:H686-H698; 1984.
Graham, M.M. Model simplification: complexity versus reduction. Circulation 72 (Suppl. IV):63–68; 1985.
Hakim, T.S.; Michel, R.P.; Chang, H.K.. Partitioning of pulmonary vascular resistance in dogs by arterial and venous occlusion. J. Appl. Physiol. 52:710–715; 1982.
House, S.D.; Johnson, J.C. Microvascular pressure in venules of skeletal muscle during arterial pressure reduction. Am. J. Physiol. 250:H838-H845; 1986.
Jones, R.D.; Berne, R.M., Autoregulation: Factors affecting vascular resistance in isolated, perfused skeletal muscle. In: Hudlicka, K., ed. Circulation in Skeletal Muscle. London: Pergamon Press; 1968.
Kajiya, F.; Tsujioka, K.; Goto, M.; Wada, Y.; Chen, X-L.; Nakai, M.; Tadaoka, S.; Hiramatsu, O.; Ogasawara, Y.; Mito, K.; Tomonaga, G. Functional characteristics of intramyocardial capacitance vessels during diastole in the dog. Circ. Res. 58:476–485; 1986.
Langewouters, G.J.; Wesseling, K.H.; Goedhard, W.J.A. The static elastic properties of 45 human thoracic and 20 abdominal aortas and the parameters of a new model. J. Biomech. 17:425–435, 1984.
Lipowsky, H.H.; Kovalcheck, S.; Zweifach, B.W. The distribution of blood rheological parameters in the microvasculature of the cat mesentery. Circ. Res. 43:738–749; 1978.
Marquardt, D.W. An algorithm for least squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 11:431–441; 1963.
Meninger, R.P.; Baker, C.H. Vascular and extravascular volume changes due to elevated venous pressure (38607). Proc. Soc. Exp. Biol. Med. 148:669–674; 1975.
Moreno, A.H.; Katz, A.L.; Gold, L.D.; Reddy, R.V., Mechanics of distension of dog veins and other very thin-walled tubular structures. Circ. Res. 27:1069–1080; 1970.
Myrhage, R.; Eriksson, E. Arrangement of the vascular bed in different types of skeletal muscles. In: Hammersen, F.; Messmer, K., eds. Skeletal Muscle Microcirculation. Progr. Appl. Microcirc., Basel: Karger, Vol. 5 (1–4); 1984.
Nichol, J.; Girling, F.; Jerrard, W.; Claxton, E.B.; Burton, A.C. Fundamental instability of the small blood vessel and critical closing pressure in vascular beds. Am. J. Physiol. 164:330–344; 1951.
Osol, G.; Halpern, W. Myogenic properties of cerebral blood vessels from normotensive and hypertensive rats. Am. J. Physiol. 249:H914-H921; 1985.
Permutt, S.; Riley, R.L. Hemodynamics of collapsible vessels with tone: the vascular waterfall. J. Appl. Physiol. 18:924–932; 1963.
Saint-Felix, D.; Demoment, G. Pressure-flow relationship in the left canine coronary artery: study of linearity and stationarity using a time domain representation and estimation methods. Med. Biol. Eng. Comp. 10:231–239; 1982.
Salotto, A.G.; Muscurella, L.F.; Melbin, J.; Li, J.K.J.; Noordergraaf, A. Pressure pulse transmission into vascular beds. Microvasc. Res. 32:152–163; 1986.
Sato, T.; Yamashiro, S.M.; Grodins, F.S. Measurement of peripheral vascular properties by a frequency response methods. Am. J. Physiol. 220:1640–1650; 1971.
Schmid-Schönbein, H. Critical closing pressure or yield shear stress as the cause of disturbed peripheral circulation? Acta Chirurgica Scan. Suppl. 465:10–19; 1976.
Schmid-Schönbein, G.W.; Firestone, G.; Zweifach, B.W. Network anatomy of arteries feeding the spinotrapezius muscle in normotensive and hypertensive rats. Blood Vessels 23:34–49; 1986.
Sherman, I.A. Interfacial tension effects in the microvasculature. Microvasc. Res. 22:296–307; 1981.
Sipkema, P.; Westerhof, N. Mechanics of a thin walled collapsible microtube. Ann. Biomed. Engng. 17:203–217; 1989.
Skalak, T.C. A mathematical hemodynamic network model of the microcirculation in skeletal muscle, using measures blood vessel distensibility and topology. San Diego, CA: University of California; 1984. PhD. dissertation.
Skalak, T.C.; Schmid-Schönbein, G.W. The microvasculature in skeletal muscle. IV. A model of the capillary network. Microvasc. Res. 32:333–347; 1986.
Smiesko, V. Unidirectional rate sensitivity component in local control of vascular tone. Pfluegers. Arch, 327:324–336; 1971.
Sobin, S.S.; Fung, Y.C. Elasticity of the pulmonary alveolar sheet. Circ. Res. 30:451–469; 1972.
Stoer, J.; Bulirsch, R. Introduction to numerical analysis. Berlin, Heidelberg: Springer Verlag, 2nd ed.; 1983.
Van Dijk, L.C.; Krams, R.; Sipkema, P.; Westerhof, N. Changes in the coronary pressure-flow relation after transition from blood to Tyrode perfusion. Am. J. Physiol. 255:H476-H482; 1988.
Whittaker, S.R.F.; Winton, F.R. The apparent viscosity of blood flowing in the isolated hindlimb of the dog, and its variation with corpuscular concentration. J. Physiol. 78:339–369; 1933.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Braakman, R., Sipkema, P. & Westerhof, N. A dynamic nonlinear lumped parameter model for skeletal muscle circulation. Ann Biomed Eng 17, 593–616 (1989). https://doi.org/10.1007/BF02367465
Issue Date:
DOI: https://doi.org/10.1007/BF02367465