Abstract
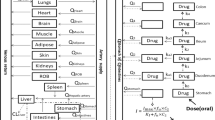
Bumetanide, 2, 8, and 20 mg/kg, was administered both intravenously and orally to determine the pharmacokinetics and pharmacodynamics of bumetanide in rats (n=10–12). The absorption of bumetanide from various segments of GI tract and the reasons for the appearance of multiple peaks in plasma concentrations of bumetanide after oral administration were also investigated. After iv dose, the pharmacokinetic parameters of bumetanide, such ast 1/2 (21.4, 53.8 vs. 127 min),CL (35.8, 19.1 vs. 13.4 ml/min per kg),CL NR (35.2, 17.8 vs. 12.6 ml/min per kg) andV SS (392, 250 vs. 274 ml/kg) were dose-dependent at the dose range studied. It may be due to the saturable metabolism of bumetanide in rats. After iv dose, 8-hr urine output per 100g body weight increased significantly with increasing doses and it could be due to significantly increased amounts of bumetanide exreted in 8-hr urine with increasing doses. The total amount of sodium and chloride exreted in 8-hr urine per 100g body weight also increased significantly after iv dose of 8 mg/kg, however, the corresponding values for potassium were dose-independent. After oral administration, the percentages of the dose excreted in 24-hr urine as unchanged bumetanide were dose-independent. Bumetanide was absorbed from all regions of GI tract studied and approximately 43.7, 50.0, and 38.4% of the orally administered dose were absorbed between 1 and 24 hr after oral doses of 2, 8, and 20 mg/kg, respectively. Therefore, the appearance of multiple peaks after oral administration could be mainly due to the gastric emptying patterns. The percentages of bumetanide absorbed from GI tract as unchanged bumetanide for up to 24 hr after oral doses of 2, 8, and 20 mg/kg (96.2, 95.4 vs. 98.2%) were not significantly different, suggesting that the problem of precipitation of bumetanide in acidic gastric juices or dissolution may not contribute significantly to the absorption of bumetanide after oral administration. Urine output per 100g body wt increased at oral doses of 8 and 20 mg/kg.
Similar content being viewed by others
References
M. Imai. Effect of bumetanide and furosemide on the thick ascending limb of Henle's loop of rabbits and rats perfused in vitro.Eur. J. Pharmacol. 41:409–416 (1977).
R. A. Branch, P. R. Read, D. Levine, E. E. Vander, J. Shelton, W. Rupp, and L. E. Ramsay. Furosemide and bumetanide: A study of responses in normal English and German subjects.Clin. Pharmacol. Ther. 19:538–545 (1976).
K. H. Olesen, B. Sigurd, E. Steiners, and A. Leth. Bumetanide, a new potent diuretic.Acta. Med. Scand. 193:119–131 (1973).
S. C. Halladay, D. E. Carter, and I. G. Sipes. A relationship between the metabolism of bumetanide and its diuretic activity in the rat.Drug Metab. Dispos. 6:45–49 (1978).
M. P. Magnussen and E. Eilertsen. Species differences in the diuretic activity and metabolism of bumetanide.Naunyn Schmiedeberg's Arch. Pharmakol. 282 (suppl.): R 61 (1974).
D. C. Brater, P. Chennavasin, B. Day, A. Burdette, and S. Andreason. Bumetanide and furosemide.Clin. Pharmacol. Ther. 34:207–213 (1983).
P. J. Pentikäinen, A Penttilä, P. J. Neuvonen and G. Gothoni, Fate of14C-bumetanide in man.Br. J. Clin. Pharmacol. 4:39–44 (1977).
P. J. Pentikäinen, P. J. Neuvonen, M. Kekki, and A. Penttilä. Pharmacokinetics of intravenously administered bumetanide in man.J. Pharmacokin. Biopharm. 8:219–228 (1980).
S. J. Kolis, T. H. Williams, and M. A. Schwarts. Identification of the urinary metabolites of14C-bumetanide in the rat and their excretion by rats and dogs.Drug Metab. Dispos. 4:169–176 (1976).
A. Ward and R. C. Heel. Bumetanide: A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use.Drugs 28:426–464 (1984).
D. E. Smith and H. S. H. Lau. Determinants of bumetanide response in the dog: Effect of probenecid.J. Pharmacokin. Biopharm. 11:31–46 (1983).
H. S. H. Lau, M. L. Hyneck, R. R. Berardi, R. D. Swarts, and D. E. Smith. Kinetics, dynamics, and bioavailability of bumetanide in healthy subjects and patients with chronic renal failure.Clin. Pharmacol. Ther. 39:635–645 (1986).
S. C. Halladay, I. G. Sipes, and D.E. Carter. Diuretic effect and metabolism of bumetanide in man.Clin. Pharmacol. Ther. 22:179–187 (1977).
A. A. Holazo, W. A. Colburn, J. H. Gustafson, R. L. Young, and M. Parsonet. Pharmacokinetics of bumetanide following intravenous, intramuscular and oral administration to normal subjects.J. Pharm. Sci. 73:1108–1113 (1984).
H. W. Chang, E. J. Yoon, M. G. Lee, and N. D. Kim, Pharmacokinetics of drugs in blood VI: Unusual distribution and storage effect of bumetanide,Seoul Univ. J. Pharm. Sci. 13:1–7 (1988).
M. G. Lee and W. L. Chiou. Evaluation of potential causes for the incomplete bioavailability of furosemide: Gastric first-pass metabolism.J. Pharmacokin. Biopharm. 11: 623–640 (1983).
F-H. Hsu, T. Prueksaritanont, M. G. Lee, and W. L. Chiou. The phenomenon and cause of the dose-dependent oral absorption of chlorothiazide in rats: Extrapolation to human data based on the body surface area concept.J. Pharmacokin. Biopharm. 15:369–386 (1987).
Y. M. Choi, S. H. Lee, S. H. Jang, and M. G. Lee. Effects of phenobarbital and 3-methylcholanthrene pretreatment on the pharmacokinetics and pharmacodynamics of bumetanide in rats.Biopharm. Drug Dispos. 12:311–324 (1991).
D. E. Smith. High performance liquid chromatographic assay for bumetanide in plasma and urine.J. Pharm. Sci. 71:520–523 (1982).
G. Lam and W. L. Chiou. Arterial and venous blood sampling in pharmacokinetic studies; Propranolol in rabbits and dogs.Res. Commun. Chem. Pathol. Pharmacol. 33:33–48 (1981).
W. L. Chiou. Critical evaluation of potential error in pharmacokinetic studies using the linear trapezoidal rule method for the calculation of the area under the plasma level-time curve.J. Pharmacokin. Biopharm. 6:539–546 (1978).
M. Gibaldi and D. Perrier.Pharmacokinetics, 2nd ed., Marcel Dekker, New York, 1982.
M. L. Chen, G. Lam, M. G. Lee, and W. L. Chiou. Arterial and venous blood sampling in pharmacokinetic studies: Griseofulvin.J. Pharm. Sci. 71:1386–1389 (1982).
W. L. Chiou. New calculation method for mean apparent drug volume of distribution and application to rationale dosage regimen.J. Pharm. Sci. 68:1068–1069 (1979).
H. J. Shim, M. G. Lee, and M. H. Lee. Factors influencing the protein binding of bumetanide using an equilibrium dialysis technique.J. Clin. Pharm. Ther. 16:467–476 (1991).
L. A. Marcantonio, W. H. R. Auld, W. R. Murdock, R. Purohit, G. G. Skellern, and O. A. Howes. The pharmacokinetics and pharmacodynamics of the diuretic bumetanide in hepatic and renal disease.Br. J. Clin. Pharmacol. 15:245–252 (1983).
S. H. Ryoo, M. G. Lee, and M. H. Lee. Effect of intravenous infusion time on the pharmacokinetics and pharmacodynamics of the same total dose of bumetanide.Biopharm. Drug Dispos. (in press).
I. Bekersky and A. Popick. Metabolism of bumetanide by the isolated perfused rat kidney.Drug. Metab. Dispos. 11:512–513 (1983).
B. Odlind, B. Beermann, and B. Lindstörm Coupling between renal tubular secretion and effect of bumetanide.Clin. Pharmacol. Ther. 34:805–809 (1983).
R. A. Branch, C. J. C. Robert, M. Homeida, and D. Levine. Determination of response to furosemide in normal subjects.Br. J. Clin. Pharmacol. 4:121–127 (1977).
M. G. Lee, T. Li, and W. L. Chiou. Effects of intravenous infusion time on the pharmacokinetics and pharmacodynamics of the same total dose of furosemide.Biopharm. Drug Dispos. 7:537–547 (1986).
T. Kahn, A. M. Kaufmann, and F. L. Mac-Moune. Response to repeated furosemide administration on low chloride and low sodium intake in the rat.Clin. Sci. 64:565–572 (1983).
G. Giebisch. In M. Martines-Moldonado (ed.),Methods of Pharmacology, Vol. 4A, Plenum Press, New York, 1976, pp. 121–164.
V. S. Chungi, I. W. Dittert, and R. B. Smith. Gastrointestinal sites of furosemide absorption in rats.Int. J. Pharm. 4:27–38 (1979).
BumexR (bumetanide/Roche).Comprehensive Product Information. Professional Service Department, Roche Laboratories, Division of Hoffman-La Roche Inc. Nutley, NJ, 1983.
F. Andreason, H.E. Boetkar, and K. Lorentzen. In vitro studies in the hydrolysis of furosemide in gastrointestinal juice.Br. J. Clin. Pharmacol. 14:306–309 (1982).
J. H. Wood, A. J. Lee, and L. K. Girrettson. Periodicity during the distribution phase for drugs administered intravenously to humans.Drug. Metab. Rev. 9:119–128 (1979).
J. A. Cook, D. E. Smith, L. A. Cornish, R. M. Tarkanow, S. M. Nicklas, and M. L. Hyneck. Kinetics, dynamics and bioavailability of bumetanide of healthy subject and patients with congestive heart failure.Clin. Pharmacol. Ther. 44:487–500 (1988).
S. Kaojarern, B. Day, and D. C. Brater. The time course of delivery of furosemide into the urine: An independent determinant of overall response.Kidney Int. 22:69–74 (1982).
Author information
Authors and Affiliations
Additional information
This work was supported in part by SNU Development Foundation, 1991.
Rights and permissions
About this article
Cite this article
Lee, S.H., Lee, M.G. & Kim, N.D. Pharmacokinetics and pharmacodynamics of bumetanide after intravenous and oral administration to rats: Absorption from various GI segments. Journal of Pharmacokinetics and Biopharmaceutics 22, 1–17 (1994). https://doi.org/10.1007/BF02353407
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02353407