Abstract
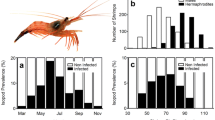
The capacity for insemination and competitive ability of small males of the partially protandrous alpheid shrimpAthanas kominatoensis Kubo which lives in symbiosis with the Japanese purple sea urchinAnthocidaris crassispina (A. Agassiz) were examined. Although male shrimps 1.5 mm in carapace length or larger produced sperm, it was not until they grew to 2.4 mm that they became functional. Males lost their insemination ability when their endopodite (or the whole) of the 2nd pleopods were amputated. The amputation of the 5th pleopods did not affect the insemination ability. The males also lost insemination ability after being infected by 2 species of Isopod ectoparasites, probably because of the transformation of the 2nd pleopods. Small males were shown to be able to achieve copulation in cohabitation experiments with amputated or parasitized large males. The total parasite infection rate in the field was highest in December and lowest in May. From August to October, when the small and large males coexisted, on the average 15.9% of large males were infected and transformed by the parasites. The presence of parasites offers the small males an opportunity for reproduction and is thought to play an important role in the evolution of protandry in this shrimp.
Similar content being viewed by others
References
Bauer, R. T. 1976 Mating behavior and spermatophore transfer in the shrimpHeptacarpus paludicora (Stimpson) (Decapoda: Caridea: Hippolytidae).J. Nat. Hist. 10: 415–440.
Bauer, R.T. 1986a Phylogenetic trends in sperm transfer and storage complexity in decapod crustaceans.J. Crust. Biol. 6: 313–325.
Bauer, R.T. 1986b Sex change and life history pattern in the shrimpThor manningi (Decapoda: Caridea): a novel case of partial protandric hermaphroditism.Biol. Bull. 170: 11–31.
Berg, A.-B.V. & P.A. Sandifer 1984 Mating behavior of the grass shrimpPalaemonetes pugio Holthuis (Decapoda, Caridea).J. Crust. Biol. 4: 417–424.
Boddeke, R., J.R. Bosschieter, & P.C. Goudswaard 1991 Sex change, mating, and sperm transfer inCrangon crangon (L.). In: R.T. Bauer & J.W. Martin (eds.)Crustacean sexual biology. pp. 164–182. Columbia University Press, New York.
Butler, T.H. 1964 Growth, reproduction, and distribution of pandalid shrimps in British Columbia.J. Fish. Res. Board Can. 21: 1403–1452.
Carpenter, A. 1978 Protandry in the freshwater shrimp,Paratya curvirostris (Heller, 1862) (Decapoda: Atyidae), with a review of the phenomenon and its significance in the Decapoda.J.R. Soc. N.Z. 8: 343–358.
Charnov, E.L. 1982 The Theory of Sex Allocation. Princeton Univ. Press, Princeton, 355 pp.
Duffy, J.E. 1992 Host use patterns and demography in a guild of tropical sponge-dwelling shrimps.Mar. Ecol. Prog. Ser. 90: 127–138.
Gherardi, F. & C. Calloni 1993 Protandrous hermaphroditism in the tropical shrimpAthanas indicus (Decapoda: Caridea), a symbiont of sea urchins.J. Crust. Biol. 13: 675–689.
Krebs, J.R. & N.B., Davies 1993 An introduction to behavioural ecology. (3rd ed.) Blackwell Scientific Publications, Oxford, 389 pp.
Nakashima, Y. 1987a Sex change in crustacea, with special reference toAthanas kominatoensis. In: A. Nakazono & T. Kuwamura (eds.)Sex change in fishes. pp. 221–248. Tokai University Press, Tokyo. (in Japanese).
Nakashima, Y. 1987b Reproductive strategies in a partially protandrous shrimp,Athanas kominatoensis (Decapoda: Alpheidae): sex change as the best of bad situation for subordinates.J. Ethol. 5: 145–159.
Policansky, D. 1982 Sex change in plants and animals.Ann. Rev. Ecol. Syst. 13: 471–495.
Shuster, S. M. 1986 Alternative reporductive behaviors: three discrete male morphs inParacerceis sculpta, an intertidal isopod from northern Gulf of California.J. Crust. Biol. 7: 318–327.
Subramonian, J. 1981 Sexual and reproductive endocrinology of Crustacea.J. Sci. Indus. Res. 40: 396–403.
Suzuki, H. 1970 Taxonomic review of four alpheid shrimps belonging to the genusAthanas with reference to their sexual phenomena.Sci. Rep. Yokohama Natl. Univ., Sect. 2, 17: 1–37.
Telecky, T. M. 1984 Alternative male reproductive strategies in the giant Malaysian prawn,Macrobrachium rosenberghii.Paci Sci. 38: 372–373.
Trivers, R. 1985 Social Evolution. The Benjamin/Cummings Publishing Company, Inc., Menlo Park, California, 462 pp.
Author information
Authors and Affiliations
About this article
Cite this article
Nakashima, Y. Can small male shrimps achieve copulation in the presence of larger ones?. J. Ethol. 13, 9–16 (1995). https://doi.org/10.1007/BF02352557
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02352557