Abstract
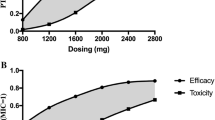
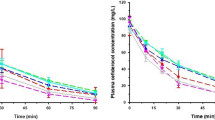
A pharmacokinetic study was done in patients undergoing continuous ambulatory peritoneal dialysis (CAPD) to establish an appropriate treatment for bacterial peritonitis. Twenty-nine CAPD patients were randomized to receive either oral ofloxacin (OFLX) 300 mg, followed by 200 mg once a day; intraperitoneal vancomycin (VCM) 30 mg/kg once a week; or intravenous imipenem/cilastatin (IPM/CS) 1000 mg once a day. Pharmacokinetic simulation models were determined according to changes of doses and duration. The time to reach the maximum plasma concentration (t max), the maximum plasma concentration (C max), the plasma elimination half-life (t 1/2), and area under the curve (AUC) were, respectively, 5.2 hours, 4.7 μg/mL, 20.4 hours and 80.5 μg/mL/h (AUC0–24) for OFLX, 7.0 h, 26.4 μg/mL, 92.0 hours, and 1641 μg/mL/h (AUC0–120) for VCM, 1.1 hour, 62.3 μg/ml, 3.76 hours, and 229.3 μg/mL/h (AUC0−1) for IPM/CS. Twenty-three of 27 (85%) strains of clinical isolates were gram-positive cocci; 20 strains (74%) consisted of staphylococci or streptococci. The MIC90 of OFLX, VCM, and IPM was 3.13, 1.56, and 0.78 μg/mL, respectively. When the minimum concentration in the dialysate during the initial 5 days was higher than the MIC, all the isolates (7 out of 7) were eradicated, when it was lower, only half of the isolates (3 out of 6) were eradicated. The simulation study suggested a significant drug accumulation in response to shortening the dosing duration.
Similar content being viewed by others
References
Nolph KD, Popovich RP, Moncrief JW. Theoretical and practical implications of continuous ambulatory peritoneal dialysis. Nephron 1978;21:117–122.
The Japanese Society for Dialysis Therapy. An overview of regular dialysis treatment in Japan (as of Dec. 31, 1994). J Jpn Soc Dial Ther 1996;29:1–22 (in Japanese).
Gokal R. Peritonitis in continuous ambulatory peritoneal dialysis. J Antimicrobial Chemother 1982;9:417–422.
Lindblad AS, Novak JW, Nolph KD. The 1987 USA National CAPD Registry Report. ASAIO J 1988;34:150–156.
Japan Society of Chemotherapy. Standard method for dilution antimicrobial susceptibility tests for bacteria (Microdilution method). Chemotherapy (Tokyo) 1990;38:103–105 (in Japanese).
Yamaoka K, Tanigawara Y, Nakagawa T, Uno T. A pharmacokinetic analysis program (MULTI) for microcomputer. J Pharmaco-Dyn 1981;4:879–885.
Morse GD, Apicella MA, Walshe JJ. Absorption of intraperitoneal antibiotics. Drug Intell Clin Pharm 1988;22:58–61.
Rubin J. Vancomycin absorption from the peritoneal cavity during dialysis-related peritonitis. Perit Dial Int 1990;10:283–285.
Rubin J, McFarland S, Hellems EW, Bower JD. Peritoneal dialysis during peritonitis. Kidney Int 1981;19:460–464.
Rottembourg J, Gahl G, Poignet JL. Severe abdominal complications in patients undergoing continuous ambulatory peritoneal dialysis. Proc Eur Dial Transplant Assoc 1983;20:236–242.
Bradley JA, Hamilton DN, McWhinnie DL, Briggs JD, Jumor BJR. Sclerosing peritonitis after CAPD (letter). Lancet 1983;2:572–573.
Paton TW, Cornish WR, Manuel MA, Hardy BG. Drug therapy in patients undergoing peritoneal dialysis: clinical pharmacokinetics considerations. Clin Pharmacokinet 1985;10:404–426.
Graevenitz A, Amsterdam D. Microbiological aspects associated with continuous ambulatory peritoneal dialysis. Clin Microbiol Rev 1992;5:36–48.
Peterson PK, Matzke G, Keane WF. Current concepts in the management of peritonitis in patients undergoing continuous ambulatory peritoneal dialysis. Rev Infect Dis 1987;9:604–612.
Nikolaidis P. Newer quinolones in the treatment of continuous ambulatory peritoneal dialysis (CAPD) related infections. Perit Dial Int 1990;10:127–133.
Janknegt R. CAPD peritonitis and fluoroquinolones: a review. Perit Dial Int 1991;11:48–58.
Janknegt R, Nube MJ. A simple method for predicting drug clearances during CAPD. J Antimicrob Chemother 1990;26 (suppl D):7–29.
Fleming LW, Philips G, Moreland TA, Stewart WK, Scott AC. Oral ciprofloxacin for treatment of peritonitis in patients on CAPD. Rev Infect Dis 1989;5(suppl 11):1294–1296.
Passlick J, Wonner R, Keller E, Essers L, Grabensee B. Single- and multiple-dose kinetics of ofloxacin in patients on continuous ambulatory peritoneal dialysis (CAPD). Perit Dial Int 1989;9:267–272.
Chan MK, Chau PY, Chan WWN. Ofloxacin pharmacokinetics in patients on continuous ambulatory peritoneal dialysis. Clin Nephrol 1987;28:277–280.
Chan MK, Chau PY, Chan WWN. Oral treatment of peritonitis in CAPD patients with two dosage regimens of ofloxacin. J Antimicrobial Chemother 1988;22:371–375.
Krogstad DJ, Moellering JC, Greenblatt DJ. Single dose kinetics of intravenous vancomycin. J Clin Pharm 1980;20:197–201.
Bunke C, Aronoff G, Brier M, Sloan R, Luft F. Pharmacokinetics of vancomycin in continuous ambulatory peritoneal dialysis. Clin Pharmacol Ther 1982;33:263.
Garrels JC, Peterie JD. Vancomycin and the “red man's syndrome” (letter). N Engl J Med 1985;312:245.
Morse GD, Farolino DF, Apicella MA, Walshe JJ. Comparative intraperitoneal and intravenous vancomycin pharmacokinetics during continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother 1987;31:173–177.
Morse GD, Narin DK, Walshe JJ. Once weekly intraperitoneal therapy for gram-positive peritonitis. Am J Kidney Dis 1987;10:300–305.
Rogers JD, Meisinger MAP, Ferber F, Calandra GB, Demetriades JL, Bland JA. Pharmacokinetics of imipenem and cilastatin in volunteers. Rev Infect Dis 1985;7:S435–446.
Calandra GB, Wang C, Aziz M, Brown KR. The safety profile of imipenem/cilastatin: worldwide clinical experience based on 3470 patients. J Antimicrob Chemother 1986;18:S193–202.
Somani P, Freimer EH, Gross ML, Higgins Jr JT. Pharmacokinetics of imipenem-cilastatin in patients with renal insufficiency undergoing continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother 1988;32:530–534.
Chan CY, Lai KN, Lam AW, Li PKT, Chung WWM, French GL. Pharmacokinetics of parenteral imipenem/cilastatin in patients on continuous ambulatory peritoneal dialysis. J Antimicrob Chemother 1991;27:225–232.
Author information
Authors and Affiliations
About this article
Cite this article
Oguchi, Ki. Pharmacokinetic study of antimicrobial agents in patients undergoing continuous ambulatory peritoneal dialysis. J Infect Chemother 2, 167–178 (1996). https://doi.org/10.1007/BF02351570
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02351570