Abstract
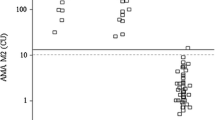
Sera from 14 normal control subjects, 30 patients with alcoholic liver diseases (fatty liver,n=8; hepatitis,n=13; liver cirrhosis,n=9), 7 controls with chronic hepatitis B, and 8 controls with chronic hepatitis C were masured for their concentrations of antibodies against HepG2 membrane protein by a binding assay utilizing125I-labeled protein A. When the cut-off level was set as the mean value plus 2 SD of normal control subjects, the incidence of positivity was 75%, 69.2%, and 77.8% in patients with alcoholic fatty liver, alcoholic hepatitis, and alcoholic cirrhosis, respectively. Both the mean serum antibody values and the positive incidence were significantly higher in patients with alcoholic liver diseases than in either the normal controls or in the control patients with chronic hepatitis. Sodium dodecylsulfate polyacrylamide gel electrophoresis of125I-labeled HepG2 membrane protein precipitated with IgG from patients with alcoholic liver diseases revealed an immunoreactive band at a molecular weight of 78 000 daltons (gp78). The antibody activity remained after immunoabsorption by human liver-specific lipoprotein (LSP) but decreased when HepG2 cells were pre-treated with trypsin or neuraminidase. Consequently, gp78 appears to be a glycoprotein distinct from LSP, and is specifically recognized by IgG from patients with alcoholic liver diseases. This assay may provide a new system to measure autoantibody to hepatocytes in alcoholic liver diseases.
Similar content being viewed by others
References
Wiel AVD, Schuurman HJ, Kater L. Alcoholic liver disease: An IgA-associated disorder. Scand J Gastroenterol 1987;22:1025–1030.
McClain CJ Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology 1989; 9:349–351.
Martinez F, Abril ER, Eamest DL, et al. Ethanol and cytokine secretion. alcohol 1992;9:455–458.
Ruiz AD, Perez JLS, Martinez GL, et al. Tumor necrosis factor, interleukin-1 and interleukin-6 in alcoholic cirrhosis. Alcohol Alcohol 1993;28:319–323.
Cochrane AMG, Moussouros A, Portmann B, et al. Lymphocyte cytotoxicity for isolated hepatocytes in alcoholic liver disease. Gastroenterologt 1977;72:918–923.
Tsuchimoto K: Detection of antibody and antibody-dependent cell-mediated cytotoxicity (ADCC) against Chang liver cell in alcoholic liver disease (in Japanese with English abstract). Nippon Shokakibyo Gakkai Zasshi (Jpn J Gastroenterol) 1982; 79:1581–1588.
Perperas A, Tsantoulas D, Portmann B et al. Autoimmunity to a liver membrane lipoprotein and liver damage in alcoholic liver disease. Gut 1981;22:149–152.
MacSween RNM, Anthony RS, Farquharson M. Antibodies to alcohol-altered hepatocytes in patients with alcoholic liver disease. Lancet 1981;II:803–804.
Izumi N, Sato C, Hasumura Y, et al. Serum antibodies against alcohol-treated rabbit hepatocytes in patients with alcoholic liver disease. Clin Exp Immunol 1985;61:585–592.
Crosley IR, Neuberger J, Davis M, et al. Ethanol metabolism in the generation of new antigenic determinants on liver cells. Gut 1986;27:186–189.
Zinneman HH. Autoimmune phenomena in alcoholic cirrhosis. Am J Dig Dis 1975;20:337–345.
Nelson-Rees WA, Flandemeyer RR. HeLa-like marker chromosomes and type-A variant glucose-6-phosphate dehydrogenase isoenzymes in human cell cultures producing Mason-Pfizer monkey virus-like particles. Science 1976;191:96–98.
Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science 1980;209:497–499.
Kumai R, Mogi Y, Kohgo Y. Suppressive effect of ethanol on the expression of asialoglycoprotein receptors in a human hepatoma cell line (in Japanese with English abstract). Sapporo Igaku Zasshi (Sapporo Med J) 1992;61:211–220.
Leevy CM, Tygstrup N. Standardization of nomenclature, diagnostic criteria and diagnostic methology for diseases of the liver and biliary tract. Basel: Karger, 1976; 1–15.
Greenwood FC, Hunter, WM, Glover JS. The preparation of131I-labeled human growth hormone of high specific radioactivity. Biochem J 1963;89:114–123.
McFarlane IG, Wojcicka BM, Zucker GM, et al. Purification and characterization of human liver-specific membrane lipoprotein (LSP). Clin Exp Immunol 1977;27:381–390.
Fairbanks G, Steck TL, Wallach DFH. Electrophoretic analysis of the major polypeptide of the human erythrocyte membrane. Biochemistry 1971;10:2606–2617.
Wiedmann KH, Bartholomew TC, Brown DJ, et al. Liver membrane antibodies detected by immunoradiometric assay in acute and chronic virus-induced and autoimmune liver disease. Hepatology 1984;4:199–204.
Ramadori G, Lenzi M, Dienes HP, et al. Binding properties of mechanically and enzymatically isolated hepatocytes for IgG and C3 Liver 1983;3:358–368.
Lenzi M, Preda P, Bianchi FB, et al. Mechanically isolated hepatocytes are unsuitable to detect antibodies directed against plasma membrane determinants. Liver 1985;5:212–220.
Gerken G, Manns M, Ramadori G. et al. Liver membrane autoantibodies in chronic active hepatitis. J Hepatol 1987;5:65–74.
Schwartz AL, Bolognesi A, Fridovich SE. Recycling of the asialoglycoprotein receptor and the effect of lysosomotropic amines in Hepatoma cells. J Cell Biol 1984;98:732–738.
Yokoyama H, Ishii H, Nagata S, et al. Experimental hepatitis induced by ethanol after immunization with acetaldehyde adducts. Hepatology 1993;17:14–19.
Swanson NR, Reed WD, Yarred LJ, et al. Autoantibodies to isolated human hepatocyte plasma membranes in chronic active hepatitis. II. Specificity of antibodies. Hepatology 1990;11:613–621.
McFarlane BM, McSorley CG, McFarlane IG, et al. A radioimmunoassay for detection of circulating antibodies reacting with the hepatic asialoglycoprotein receptor protein. J Immunol Methods 1985;77:219–228.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Numata, T., Kato, J., Kohgo, Y. et al. Autoantibody against a 78 kDa membrane protein of HepG2 cell in the sera of patients with alcoholic liver diseases. J Gastroenterol 30, 751–757 (1995). https://doi.org/10.1007/BF02349642
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02349642