Abstract
Background
The antitumor effects of adoptive immunotherapy in combination with chemoradiotherapy were investigated in patients with oral squamous cell carcinoma.
Methods
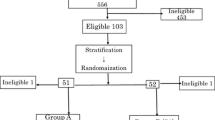
Inductive chemoradiotherapy with peplomycin, 5-fluorouracil and60Co was given to 56 patients [CRI(−)group]. A local injection of adoptive lymphokine-activated killer (LAK) cells (≈2×108 cells) and small doses of interleukin-2(≈3×105 U) and interferon-gamma (≈2×105U) was given to 40 other patients in combination with the chemoradiotherapy [CRI(+) group].
Results
Clinically, CR, PR, and LR were observed in 15 (37.5%), 24 (60.0%), and 1 (2.5%) of the patients in the CRI (+) group, respectively; and in 14 (25.0%), 38 (67.9%), and 4 of the patients (7.1%) in the CRI (−) group. The histopathological effects were correlated with the tumor remission rate, with lethal degeneration (grades III and IV), and prominent degeneration (grade IIB) in the tumor cells noted in 20 (50.0%) and 16 (40.0%) of the CRI(+) patients, respectively; and in 21 (37.5%) and 29 (51.8%) of the CRI (−) patients. Immunohistochemically, a prominent decrease of proliferating cell nuclear antigenpositive cells with a reciprocal increase of LeY-positve cells was induced by the chemoradioimmunotherapy. DNA fragmentation was observed in the mutant type p53-negative tumors in the CRI(+) group.
Conclusion
Adoptive immunotherapy with LAK cells and cytokines in combination with chemoradiotherapy induces advantageous anticancer effects resulting from necrosis and apoptosis.
Similar content being viewed by others
References
Teicher BA, Herman TS, Holden SA. Combined modality therapy with bleomycin, hyperthermia, and radiation. Cancer Res 1988;48:6291–6297.
Morikawa K, Fan D, Denkins YM, Levin B, Gutterman JU, Walker SM, Fidler IJ. Mechanisms of combined effects of γ-interferon and 5-fluorouracil on human colon cancers implanted into nude mice. Cancer Res 1989;49:799–805.
Gobji K, Macda S, Sugiyama T, Ishigami J, Kamidono S. Enhanced inhibition of anticancer drugs by human recombinant γ-interferon for human renal cell carcinoma in vitro. J Urol 1987;137:539–543.
Ervin TJ, Clark JR. An analysis of induction and adjuvant chemotherapy in the multidisciplinary treatment of squamous cell carcinoma of the head and neck. J Clin Oncol 1987;5:10–20.
Adelstein DJ, Sharan VM, Earle AS, Shah AC, Vlastou C, Haria CD, Damm C, Carter SG, Hines JD. Simultaneous versus sequential combined technique therapy for squamous cell head and neck cancer. Cancer 1990; 65:1685–1691.
Reboul F, Vincent P, Chauvet B, Brewer Y, Felix-faure C, Taulelle M, Shrieve DC. Radiation therapy with concomitant continuous infusion cisplatin for unresectable nonsmall cell lung carcinoma. Int J Radiat Oncol Biol Phys 1994;28:1251–1256.
Osaki T, Yoneda K, Hirota J, Yamamoto T, Ueta E. Function-preserving surgery after induction therapy in tongue and floor of the mouth carcinomas. Asian J Oral Maxillofac Surg 1994;6:127–135.
Miyashita T, Reed JC. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood 1993;81:151–157.
Akagi Y, Ito K, Sawada S. Radiation-induced apoptosis and necrosis in Molt-4 cells: a study of dose-effect relationships and their modification. Int J Radiat Biol 1993;64:47–56.
Koller CA, Miller DM. Preliminary observation on the therapy of the myeloid blast phase of chronic granulocytic leukemia with plicamycin and hydroxyurea. N Engl J Med 1986;315:1433–1438.
Wangerheim KH, Howard A. Different modes of cell sterilization: cell killing and early differentiation. Radiat Res 1978;73:288–302.
Sato M. Treatment of oral cancer. Asian J Oral Maxillofac Surg 1993;5:87–99.
Shov KA, MacPhail HS, Marples B. The effect of radiosensitizers on the survival response of hypoxic mammalian cells: the low X-ray dose region, hypersensitivity and induced radioresistance. Radiat Res 1994;138(S):113–116.
Ulrich K, Peter S, Wolfgang T, Bernadett B, Frank G, Johannes G, Gunther WK, Peter M, Stefon S, Werner H. Regional administration of lymphokine-activated killer cells can be superior to intravenous application. Cancer 1992;69:2172–2175.
Mavligit GM, Zukiwski AA, Guttermann JU, Salem P, Charnsangavej C, Wallace S. Splenic versus hepatic artery infusion of interleukin-2 in patients with liver metastases. J Clin Oncol 1990;8:319–324.
Duke RC, Chervenak R, Cohen JJ. Endogenous endonuclease induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci USA 1983;80:6361–6365.
Duke RC, Cohen JJ, Chervenak R. Differences in target cell DNA fragmentation induced by mouse cytotoxic T lymphocytes and natural killer cells. J Immunol 1986;137:1442–1447.
Sato M, Yoshida H, Kaji R, Okamoto M, Iga H, Kawamata H, Kobayashi S, Aladib W, Yanagawa T, Takegawa Y. Induction of bone formation in an adenoid cystic carcinoma of the maxillary sinus by adoptive immunotherapy involving intra-arterial injection of lymphokine-activated killer cells and recombinant interleukin-2 in combination with radiotherapy. J Biol Response Mod 1990;9:329–334.
Yoneda K, Yamamoto T, Ueta E, Osaki T. Induction of lymphokine-activated killer cells with low-dose interleukin-2 and interferon-γin oral cancer patients. J Clin Immunol 1992;12:289–299.
Salup RR, Wiltrout RH. Adjuvant immunotherapy of established murine renal cancer by interleukin-2-stimulated cytotoxic lymphocytes. Cancer Res 1986;46:3358–3363.
Stratton JA, Braly PS, Rettenmaier MA, diSaia PJ. Depressed natural killer cell activity in tumor-bearing rats: effect of immunotherapy and cytoreductive chemotherapy. Clin Immunol Immunopathol 1983;28:170–176.
Leroux J, Mercier G, Oth D. Enhancement of murine lymphoma cell lyseability by CTL and LAK cells, treatments with mitomycin C and adriamycin. Int J Immunopharmacol 1986;8:369–375.
Yoneda K, Osaki T, Yamamoto T, Ueta E. Effects of tumor necrosis factor-alpha (TNF-α), II-1β and monocytes on lymphokine-activated killer (LAK) induction from natural killer (NK) cells and T lymphocytes. Clin Exp Immunol 1993;93:229–236.
Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207–214.
Shimosato Y, Ohoshi S, Baba K. Histological evaluation of effects of radiotherapy and chemotherapy for carcinomas. Jpn J Clin Oncol 1971;1:19–35.
Osaki T, Ueta E, Yoneda K, Hirota J, Yamamoto T. Prophylaxis of oral mucositis associated with chemoradiotherapy for oral carcinoma by azelastine hydrochloride (Azeptin) with other antioxidants. Head & Neck 1994;16:331–339.
Osaki T, Hirota J, Yoneda K, Yamamoto T, Ueta E. Induction chemoradiotherapy and conservative surgery to oral carcinomas with the aim of minimum oral dysfunction. J Jpn Soc Oral Tumor 1991;3:198–212.
Osaki T, Hirota J, Yoneda K, Yamamoto T, Ueta E. Distribution of surviving tumor cells after chemoradiotherapy in tongue and floor of mouth carcinomas. Head & Neck 1994;16:218–226.
Lesuffleur T, Kornowski A, Luccioni C, Muleris M, Barbat A, Beaumatin J, Dussaulx E, Dutrillaux B, Zweibaum A. Adaptation to 5-fluorouracil of the heterogeneous human colon tumor cell line HT-29 results in the selection of cells committed to differentiation. Int J Cancer 1991;49:721–730.
Barni S, Bernocchi G, Scherini E, Mares V. Growth and polyploidization of the liver of early postnatal rats treated with bleomycin. Cell Biol Int Rep 1985;9:993–1001.
Tomida M, Yamamoto Y, Hozumi M. Stimulation by interferon of induction of differentiation of mouse myeloid leukemic cells. Cancer Res 1980;40:2919–2924.
Sandvig S, Laskay T, Anderson J, Deley M, Anderson U. Gamma-interferon is produced by CD3+ and CD3− lymphocytes. Immunol Rev 1987;97:51–65.
Heusel JW, Wesselschmidt RL, Shresta S, Russell JH, Ley TJ. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell 1994;76:977–987.
Nakajima H, Henkart PA. Cytotoxic lymphocyte granzymes trigger a target cell internal disintegration pathway leading to cytolysis and DNA breakdown. J Immunol 1994;152:1057–1063.
Sheetov MM, Cheng DLW, Kong L, Shu WP, Sassaroli M, Droller MJ, Liu BCS. LAK cell mediated apoptosis of human bladder cancer cells involves a pH-dependent endonuclease system in the cancer cell: possible mechanism of BCG therapy. J Urol 1995;154:269–274.
Shresta S, MacIvor DM, Heusel JW, Russel JH, Ley TJ. Natural killer and lymphokine-activated killer cells require granzyme B for the rapid induction of apoptosis in susceptible target cells. Proc Natl Acad Sci USA 1995;92: 5679–5683.
Higuchi M, Aggarwal BB. Differential roles of two types of the TNF receptor in TNF-induced cytotoxicity, DNA fragmentation, and differentiation. J Immunol 1994;152:4017–4025.
Cotter TG, Glynn JM, Echeverri F, Green DR. The induction of apoptosis by chemotherapeutic agents occurs in all phases of the cell cycle. Anticancer Res 1992;12:773–780.
Mangan D, Robertson B, Wahl SM. IL-4 enhances programmed cell death (apoptosis) in stimulated human monocytes. J Immunol 1992;148:1812–1816.
Mangan D, Wahl SM. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines. J Immunol 1991;147:3408–3412.
Selmaj K, Raine CS, Farooq M, Norton WT, Brosman CF. Cytokine cytotoxicity against oligodendrocytes. Apoptosis induced by lymphotoxin. J Immunol 1991;147:1522–1529.
Bhalla K, Tang C, Ibrado AM, Grant S, Tourkina E, Holladay C, Hughes M, Mahoney ME, Huang Y. Granulocyte macrophage colony-stimulating factor/interleukin-3 fusion protein (pIXY321) enhances highdose Ara-C-induced programmed cell death or apoptosis in human myeloid leukemia cells. Blood 1992;80:2883–2890.
Weller M, Frei K, Groscurth P, Krammer PH, Yonekawa Y, Fontana A. Anti-Fas/APO-1 antibody-mediated apoptosis of cultured human glioma cells. Induction and modulation of sensitivity by cytokines. J Clin Invest 1994;94:954–964.
Shoji Y, Uedono Y, Ishikura H, Takeyama N, Tanaka T. DNA damage induced by tumour necrosis factor-α in L929 cells is mediated by mitochondrial oxygen radical formation. Immunology 1995;84:543–548.
Okamoto M, Kaji R, Kasetani H, Yoshida H, Moriya Y, Saito M, Sato M. Purification and chracterization of interferon-gamma-inducing molecule of OK-432, a penicillin-killed streptococcal preparation, by monoclonal antibody neutralizing interferon-gamma inducing activity of OK-432. J Immunother 1993;13:232–242.
Sakakura C, Takahashi T, Hagiwara A, Yamane T, Sawai S, Okano S, Ozaki K, Sasaki S, Tsujimoto H. Enhancement of LAK-like activity and cytokine induction in regional lymph nodes and spleen cells of mice after intralymphnodal injection of OK-432, a killed streptococcal preparation. Anticancer Drugs 1993;4:381–388.
Author information
Authors and Affiliations
About this article
Cite this article
Yamamoto, T., Yoneda, K., Ueta, E. et al. Combination adoptive immunotherapy with chemoradiotherapy in oral carcinomas. Int J Clin Oncol 1, 150–156 (1996). https://doi.org/10.1007/BF02348381
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02348381