Abstract
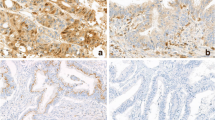
The biological characteristics associated with the morphological diversity of colorectal cancers were investigated to elucidate the causes of this diversity. We examined the proliferative and infiltrating activity of tumor cells, indicated by the mean number of Ag nucleolar organizer region associated proteins (NORs) per nucleus (MNA) and the immunohistochemical response to cathepsin B(CB), in various morphological types of early and advanced colorectal cancers. We examined 73 colorectal cancers obtained by endoscopic and surgical resection. MNA values for sessile and flat-elevated cancers were greater than the values for pedunculate, subpedunculate, and flat-or-depressed early cancers (sessile,P<0.05). In advanced cancers invading the muscularis propria, protruding cancers showed significantly higher MNA values than small ulcerative cancers (P<0.01). CB expression increased significantly with the progression of colorectal cancers (P<0.01), but was not related to morphological diversity in early and advanced cancers. In both sessile and flat cancers, CB expression was higher in moderately differentiatiated than in well differentiated adenocarcinomas. These results indicate that, in colorectal cancers, protruding early cancers without stalks and protruding advanced cancers have higher proliferative activity than pedunculate or flat early cancers and small ulcerative advanced cancers, respectively, and that CB expression is not associated with morphological diversity, but with depth of invasion and histological differentiation.
Similar content being viewed by others
References
Shimoda T, Ikegami M, Fujisaki J, et al. Early colorectal carcinoma with special reference to its development de novo. Cancer 1989;64:1138–1146.
Muto T. Endoscopic and histological features of small early carcinomas of the colon with special reference to flat elevation and type IIa carcinoma (in Japanese with English abstract). Ito Cho (Stomach Intestin) 1987;22:397–406.
Tamaka S, Tatsuta S, Ohtsu N et al. Assessment for development of superficial colorectal neoplasm—endoscopic and clinicopathologic analysis of 149 submucosal invasive carcinomas (in Japanese with English abtract). Nippon Shokakibyo Gakkai Zasshi (Jpn J Gastroenterol). 1994;91:1182–1189.
Satonaka K, Nagasako K, Fujimori T, et al. The study of K-ras point mutation in superficial type of colorectal cancer (in Japanese with English abstract). Shoukakiganno Hasseito Shinten (J Jpn Res Soc Gastroenterol Cancinogenesis) 1991;3:181–184.
Yamagata S, Muto T, Uchida Y, et al. Lower incidence of K-ras codon 12 mutation in flat colorectal adenomas than in polypoid adenomas. Jpn J Cancer Res 1994;85:147–151.
Kurvink K, Konica K, Porzucek L. Acrocentric interconnections and NOR variants in human lymphocytes. Cancer Genet Cytogenet 1990;50:207–226.
Crocker J, Nar P. Nucleolar organizer regions in lymphomas. J Pathol 1987;151:111–118.
Crocker J, McGovern J. Nucleolar organiser regions in normal, cirrhotic, and carcinomatous livers, J Clin Pathol 1988;41:1044–1048.
Derenzini M, Romagnoli T, Mingazzini P, et al. Interphasic nucleolar organizer region distribution as a diagnostic parameter to differentiate benign from malignant epithelial tumors of human intestine. Virchows Arch [B] 1988;54:334–340.
Greenhaum LM, Fruton JS. Purification and properties of beef spleen cathepsin B. J Biol Chem 1957;226:173–180.
Maciewics RA, Wardale JR, Etherington DJ, et al. Immunodetection of cathepsins B and L present in and secreted from human premalignant and malignant colorectal tumour cell lines. Int J Cancer 1989;43:478–486.
Honn KV, Timar J, Rozhin J, et al. A lipoxygenase metabolite, 12-(S)-HETE, stimulates protein kinase C-mediated release of cathepsin B from malignant cells. Exp Cell Res 1994;214:120–130.
Watanabe M, Higashi T, Watanabe A, et al. Cathepsin B and L activities in gastric cancer tissue: Correlation with histological findings. Biochem Med Metabol Biol 1989;42:21–29.
Campo E, Munoz J, Miquel R, et al. Cathepsin B expression in colorectal carcinomas correlates with tumor progression and shortended patient survival. Am J Pathol 1991;145:301–309.
Japanese Research Society for Cancer of the Colon and Rectum. General rules for clinical and pathological studies on cancer of the colon, rectum and anus. 5th ed. Tokyo: Kahehara, 1994;5–6.
Nagasako K. Natural history of superficial colorectal early cancers—from superficial to ulcerated lesions (in Japanese with English abstract). Nippon Shokaki Naishikyou Gakkai Zasshi (Gastroenterol Endosc) 1993;5:289–296.
Jass JR, Sobin LH, Large intestine. World Health Organization's histological typing of intestinal tumours. 2nd ed. Berlin Heidelberg New York London Paris Tokyo Hong Kong: Springer 1989;29–40.
Proton D, Menager M, Jeannesson P, et al. Improvement in the staining and in the visualization of the argyrophilic proteins of the nucleolar organizer region at the optic level. Histochem J 1986;18:5–14.
Higashiyama M, Doi O, Kodama K, et al. Cathepsin B expression in tumour cells and laminin distribution in pulmonary adenocarcinoma. J Clin Pathol 1993;46:18–22.
Spratt JS, Ackerman LV. Small primary adenocarcinomas of the colon and rectum. JAMA 1962;179:337–362.
Adachi M, Muto T, Morioka Y, et al. Flat adenoma and flat mucosal carcinoma (IIb Type)—a new precursor of colorectal carcinoma? Dis Colon Rectum 1988;31:236–343.
Rubio C, Shetye J. Flat adenoma-adenocarcinoma sequence in the colon of rats. Dis Colon Rectum 1994;35:1300–1306.
Shimoda T. Pathological features of early colorectal carcinomas originating from superficial type (in Japanese with English abstract). Ito Cho (Stomach Intestine) 1995;30:141–147.
Raza A, Ukar K, Preisler HD. Double labeling and in vitro versus in vivo incorporation of bromodeoxyuridine in patients with acute nonlymphocytic leukemia. Cytometry 1985;6:633–640.
Mushika M, Miwa T, Suzuoki Y et al. Detection of proliferative cells in dysplasia, carcinoma in situ, and invasive carcinoma of the uterine cervix by monoclonal antibody against DNA polymerase α. Cancer 1988;61:1182–1186.
Gerdes J, Schwab U, Lemke H, et al. Production of a mouse monoclonal antibody reactive with human nuclear antigen associated with cell proliferation. Int J Cancer 1983;31:13–20.
Bravo R, Macdonald-Bravo H. Changes in the nuclear distribution of cyclin (PCNA) but not its synthesis depend on DNA replication. EMBO J 1985;655–661.
Janmohamed RMI, Murray PG, Crocker J, et al. Sequential demonstration of nucleolar organizer regions and Ki67 immunolabeling in non-Hodgkin's lymphomas. Clin Lab Haematol 1990;12:395–399.
Trere D, Pession A, Drenzini M. The siver-stained proteins of interphasic nucleolar organizer regions as a parameter of cell duplication rate. Exp Cel Res 1989;184:131–137.
Derenzini M, Pession A, Trere D. Quntity of nucleolar silverstained proteins is related to proliferating activity in cancer cells. Lab Invest 1990;63:137–145.
Reeves BR, Casey S, Honeycombe JR, et al. Correlation of differentiation and silver staining of nucleolar organizers in the promyelocytic cell line HL-60. Cancer Genet Cytogenet 1984; 13:159–166.
Mitomi H, Atari E, Uesugi H, et al. Pathological study of flat and polypoid type adenomas of the colon (in Japanese with English abstract). Nippon Daichou Koumonbyo Gakkai Zasshi (J Jpn Soc Coloproctol) 1994;47:401–412.
Yamada Y, Iwashita A. Immunohistochemical study of minute adenomas of the large intestine (in Japanese with English abstract). Ito Cho (Stomach Intestine). 1995;30:1543–1550.
Murata N. Relationship between macroscopic appearance and objective atypism of Ag-NORs in minute colorectal epithelial neoplasms (in Japanase with English abstract). Nippon Daichou Koumonbyou Gakkai Zasshi (J Jpn Soc Coloproctol). 1995; 48:281–288.
Kasumi A, Kratzer GL. Fast growing cancer of the colon and rectum. Am Surg 1992;58:383–386.
Watari J, Yokota K. Macroscopic classificztion and kinetic alterations in the superficial lesions of colorectal neoplasmas (in Japanese with English abstract). Nippon Shokakibyo Gakkai Zasshi (Jpn J Gastroenterol) 1994;91:2040–2048.
Rayter Z, Surtees P, Tildsley G, et al. The prognostic value of argyrophil nucleolar organiser regions (AgNORs) in colorectal cancer. Eur J Surg Oncol 1992;18:37–40.
Buck MR, Karustis DG, Day NA, et al. Degradation of extracellular-matrix proteins by human cathepsin B from normal and tumor tissues. Biochem J 1992;282:273–278.
Visscher DW, Sloane BF, Sameni M, et al. Clinicopathologic significance of cathepsin B immunostaining in transitional neoplasia. Mod Pathol 1994;7:76–81.
Sukoh N, Abe S, Nakajima I, et al. Immunohistochemical distributions of cathepsin B and basement membrane antigens in human lung adenocarcinoma: Association with invasion and metastasis. Virchows Arch [A]. 1994;424:33–38.
Terada T, Ohta T, Minato H, et al. Expression of pancreatic trypsinogen/trypsin and cathepsin B in Human cholangiocarcinomas and hepatocellular carcinomas. 1995;26:746–752.
Sheahan K, Shuja S, Murnane MJ. Cysteine protease activities and tumor development in human colorectal carcinoma. Cancer Res 1989;49:3809–3814.
Corticchiato O, Cajot J-F, Abrahamson M, et al. Cystatin and cathepsin B in human colon carcinoma: Expression by cell lines and matrix degradation. Int J Cancer 1992;52:645–652.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nanashima, A., Tagawa, Y., Nakagoe, T. et al. Relationship between morphological diversity and AgNORs or cathepsin B expression in colorectal cancers. J Gastroenterol 31, 646–653 (1996). https://doi.org/10.1007/BF02347611
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02347611