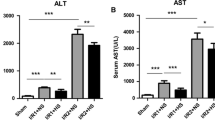
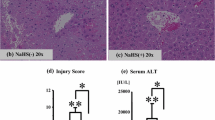
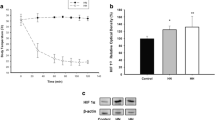
Abstract
Controversy persists as to whether reperfusion-induced injuries actually occur in the hepatocyte. The liver is the major source of glutathione, a scavenger of hydrogen peroxide. The aim of this study was to evaluate the sensitivity of the ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG) [GSH: GSSG] as an index of hepatic metabolic stress. A total of 121 rats were studied. The superior mesenteric vein (SMV) was occluded for 30 min, and this was followed by 0, 10, or 120 min of reperfusion. Total glutathione and GSSG levels in the liver, bile, and plasma were quantified, using glutathione reductase-coupled enzymatic assays. Results indicated that the hepatic GSH/GSSG ratio was maintained after an occulusion of the SMV, despite a decrease in adenosine triphosphate (ATP) level and energy charge potential. However, plasma levels of total glutathione and GSSG in the inferior vena cava increased after SMV occlusion and continued to increase after reperfusion. Biliary GSSG efflux decreased during 30-min occlusion of the SMV, and remained low even after reperfusion. The liver maintains homeostasis despite a decrease in biliary GSSG efflux, probably by secreting excess GSSG into the hepatic vein when the SMV is occluded. We conclude that the total amount of glutathione and GSSG in the plasma is directly correlated with oxidative stress in the liver.
Similar content being viewed by others
References
Bellomo G, Thor H, Mirabelli F, Richelmi P, et al. Alterations in hepatocyte cytoskeleton during the metabolism of quinones. In: Poli G, Cheeseman KH, Dianzani MU, Slater TF (eds) Free radicals in the pathogenesis of liver injury. Advances in the Biosciences, 76. 1st ed. New York: Pergamon, 1989.
Korthuis RJ, Granger DN, Townsley MI, Taylor AE. The role of oxygen-derived free radicals in ischemia-induced increases in canine skeletal muscle vascular permeability. Circ Res 1985; 57:599–609.
Di Monte D, Ross D, Bellomo G, et al. Alterations in intracellular thiol homeostasis during the metabolism of menadione by isolated rat hepatocytes. Arch Biochem Biophys Res Commun 1984;235:334–342.
Bellomo G, Orrenius S. Altered thiol and calcium homeostasis in oxidative hepatocellular injury. Hepatology 1985;5:876–882.
Ookhtens M, Hodby K, Corvasce MC, et al. Sinusoidal efflux of glutathione in the perfused rat liver. J Clin Invest 1985;75: 258–265.
Aw TY, Ookhtens M, Clement R, Kaplowitz AN. Kinetics of glutathione efflux from isolated rat hepatocytes. Am J Physiol 1986;250:G236-G243.
Akerboom TPM, Inoue M, Sies H, et al. Biliary transport of glutathione disulfide studied with isolated rat-liver canalicularmembrane vesicles. Eur J Biochem 1984;141:211–215.
Marubayashi S, Dohi K, Yamada K, Kawasaki T. Changes in the levels of endogenous coenzyme Q homologs, α-tocopherol, and glutathione in rat liver after hepatic ischemia and reperfusion, and the effect of pretreatment with coenzyme. Q10. Biochim Biophys Acta 1984;797:1–9.
Atkinson DE. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 1966;7:4030–4034.
Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione. Anal Biochem 1969;27:502–522.
Eberle D, Clarke R, Kaplowitz N. Rapid oxidation in vitro of endogenous and exogenous glutathione in bile of rats. J Biol Chem 1981;256:2115–2117.
McCoy RN, Hill KE, Ayon MA, et al. Oxidant stress following renal ischemia: Changes in the glutathione redox ratio. Kidney Int 1988;33:812–817.
Inoue M, Kinne R, Tran T, Arias IM. The mechanism of biliary secretion of reduced glutathione. Eur J Biochem 1983;134:467–471.
Akerboom TPM, Bilzer M, Sies H. The relationship of biliary glutathione disulfide efflux and intracellular glutathione disulfide content in perfused rat liver. J Biol Chem 1982;257:4248–4252.
Kaplowitz N, Eberle DE, Petrini J, et al. Factors influencing the efflux of hepatic glutathione into bile in rats. J Pharmacol Exp Ther 1983;224:141–147.
Abdalla EK, Caty MG, Guice KS, et al. Arterial levels of oxidized glutathione (GSSG) reflect oxidant stress in vivo. J Surg Res 1990;48:291–296.
Sies H, Gerstenecker C, Menzel H, Flohe L. Oxidation in the NADP system and release of GSSG from hemoglobin-free perfused rat liver during peroxidatic oxidation of glutathione by hydroperoxides. FEBS Lett 1972;27:171–175.
Stein HJ, Oosthuizen MMJ, Hinder AR, Lamprecht H. Oxygen free radicals and glutathione in hepatic ischemia/reperfusion injury. J Surg Res 1991;50:398–402.
Sies H, Brigelius R, Graf P. Hormones, glutathione status and protein s-thiolation. Adv Enzyme Regul 1987;26:175–189.
Young JD, Jones SEM, Ellory JC. Amino acid transport in human and in sheep erythrocytes. Proc R Soc Lond [Biol] 1980;209:355–375.
Rouzer CA, Scott WA, Griffith OW, et al. Glutathione metabolism in resting and phagocytizing peritoneal macrophages. J Biol Chem 1982;257:2002–2008.
Watanabe H, Bannai S. Induction of cystine transport activity in mouse peritoneal macrophages. J Exp Med 1987;165:628–640.
Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem 1986;261:2256–2263.
Jaeschke H, Smith CV, Mitchell R. Reactive oxygen species during ischemia-reflow injury in isolated perfused rat liver. J Clin Invest 1988;81:1240–1246.
Jaeschke H. Glutathione disulfide as index of oxidant stress in rat liver during hypoxia. Am J Physiol 1990;258:G499-G505.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Denno, R., Takabayashi, A., Sugano, M. et al. The ratio of reduced glutathione/oxidized glutathione is maintained in the liver during short-term hepatic hypoxia. J Gastroenterol 30, 338–346 (1995). https://doi.org/10.1007/BF02347509
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02347509