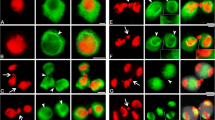
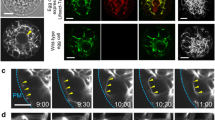
Abstract
Video microscopy and conventional or Confocal Laser Scanning Microscopy after DAPI staining and anti-α-tubulin labelling were used to study the asymmetrical division of the generative cell (GC) inGagea lutea. Pollen was cultured for up to 8 hr in a medium containing 10% poly (ethylene glycol), 3.0% to 3.8% sucrose, 0.03% casein acid hydrolysate, 15 mM 2-(N-morpholinoethane)-sulphonic acid-KOH buffer (pH 5.9) and salts. In the pollen grain, the GC had a spherical or ovoid shape and contained a fine network of intermingled microtubules. As the GC entered into the pollen tube, it assumed a cylindrical shape with a length often exceeding 250 μm. A cage of microtubules then developed around the nucleus. The presence of dense and thick microtubular bundles in front of the generative nucleus within the GC coincided with the translocation of the nucleus to the leading end of the GC. No pre-prophase band was ever detected, but a distinct prophase spindle of microtubules was formed. In some GCs a tubulin-rich dot became visible at each pole of the spindle. After nuclear envelope breakdown, the bundles of microtubules spread between the chromosomes and became oriented into parallel arrays. The spindle became shorter at metaphase, and there was no tubulin labelling at the site of the metaphase plate. At anaphase, the microtubular apparatus lost its spindle-shape and a bridge of prominent bundles of microtubules connected the two daughter nuclei. At telophase, the site of the cell plate remained unstained by the anti-α-tubulin antibody, but a distinct phragmoplast of microtubules was formed more closely to the leading nucleus, resulting in the formation of unequal sperm cells (SCs). The leading SC was up to 2.5 times smaller than the following SC and it contained a smaller or equal number of nucleoli.
Similar content being viewed by others
Abbreviations
- PEG:
-
polyetheleneglycol
- PFA:
-
paraformaldehyde
- FITC:
-
fluorescein iso-thiocyanate
- DAPI:
-
4, 6-diamidino-2-phenylindole
- CLSM:
-
confocal laser scanning microscopy
- DIC:
-
differential interference contrast
- DiOC6 (3):
-
3, 3′-dihexyloxacarbocyanine iodide
References
Aström, H., Virtanen, I. andRaudaskoski, M. 1991. Coldstability in the pollen tube cytoskeleton. Protoplasma160: 99–107.
Baskin, T.I. andCande, Z. 1990. The structure and function of the mitotic spindle in flowering plants. Annu. Rev. Plant Physiol.41: 277–315.
Chaboud, A. andPerez, R. 1992. Generative cells and male gametes: isolation, physiology, and biochemistry. Int. Rev. Cytol.140: 205–232.
Coleman, A.W. andGoff, L.J. 1985. Applications of fluorochromes to pollen biology-1. Mithramycin and 4,6-diamidino-2-phenylindole (DAPI) as vital stains and for quantitation of nuclear DNA. Stain Technol.60: 145–154.
Del Casino, C., Tiezzi, A., Wagner, V.T. andCresti, M. 1992. The organization of the cytoskeleton in generative cell and sperms ofHyacinthus orientalis. Protoplasma168: 41–50.
Derksen, J., Pierson, E.S. andTraas, J.A. 1985. Microtubules in vegetative and generative cells of pollen tubes. Eur. J. Cell Biol.38: 142–148.
Dumas, C., Knox, R.B. andGaude, T. 1985. The spatial association of the sperm cells and vegetative nucleus in the pollen grain ofBrassica. Protoplasma124: 168–174.
Gunning, B.E.S. andWick, S.M. 1985. Preprophase bands, phragmoplasts and spatial control of cytokinesis. J. Cell Sci. Suppl.2: 157–179.
LaFleur, G.J., Gross, A.E. andMascarenhas, J.P. 1981. Optimization of culture conditions for the formation of sperm cells in pollen tubes ofTradescantia. Gamete Res.4: 35–40.
Lambert, A.M. 1993. Microtubule organizing centers in higher plants. Curr. Opin. Cell Biol.5: 116–122.
Liu, Z., Bushnell, W.R. andBrambl, R. 1987. Pontentiometric cyanine dyes are sensitive probes for mitochondria in intact plant cells. Plant Physiol.84: 1385–1390.
McConchie, C.A., Jobson, S. andKnox, R.B. 1985. Computer-assisted reconstruction of the male germ unit in pollen ofBrassica campestris. Protoplasma127: 57–63.
McConchie, C.A., Hough, T. andKnox, R.B. 1987a. Ultrastructural analysis of the sperm cells of mature pollen of maize.Zea mays. Protoplasma139: 9–19.
McConchie, C.A., Russell, S.D., Dumas, C., Tuohy, M. andKnox, R.B. 1987b. Quantitative cytology of the sperm cells ofBrassica campestris andB. oleracea. Planta170: 446–452.
Miller, D.D., Callaham, D.A., Gross, D.J. andHepler, P.K. 1992. Free Ca2+ gradient in growing pollen tubes ofLilium. J. Cell Sci.101: 7–12.
Mogensen, H.L. 1992. The male germ unit: concept, composition, and significance. Int. Rev. Cytol.140: 129–147.
Murgia, M. andWilms, H.J. 1988. Three-dimensional image and mitochondrial distribution in sperm cells ofEuphorbia dulcis.In: H.J. Wilms and C.J. Keijzer, eds., Plant Sperm Cells as Tools for Biotechnology. Pudoc. Wageningen, pp. 111–112.
Oakley, B.R. 1992. γ-Tubulin: The microtubule organizer? Trends Cell biol.2: 1–5.
Palevitz, B.A. andTiezzi, A. 1992. Organization, composition, and function of the generative cell and sperm cytoskeleton. Int. Rev. Cytol.140: 149–185.
Palevitz, B.A., Liu, B. andJoshi, H.C. 1994. γ-tubulin in tobacco pollen tubes: association with generative cell and vegetative microtubules. Sex. Plant Reprod.7: 209–214.
Pandolfi, T., Pacini, E., andCalder, D.M. 1993. Ontogenesis of monad pollen inPterostylis plumosa (Orchidaceae, Neottioideae). Pl. Syst. Evol.186: 175–185.
Plerson, E.S. andCresti, M. 1992. Cytoskeleton and cytoplasmic organization of pollen and pollen tubes. Int. Rev. Cytol.140: 73–125.
Read, S.M., Clarke, A.E. andBacic, A. 1993. Requirements for division of the generative nucleus in cultured pollen tubes ofNicotiana. Protoplasma174: 101–115.
Rusche, M.L. andMogensen, H.L. 1988. The male germ unit ofZea mays: Quantitative ultra structure and three-dimensional analysis.In M. Cresti, P. Gori and E. Pacini, eds., Sexual Reproduction in Higher Plants. Springer-Verlag, Berlin, Heidelberg, pp. 221–226.
Russell, S.D. 1984. Ultrastructure of the sperm ofPlumbago zeylanica: 2. Quantitative cytology and threedimensional reconstruction. Planta162: 385–391.
Russell, S.D. 1985. Preferential fertilization inPlumbago: Ultrastructural evidence for gamete-level recognition in an angiosperm. Proc. Natl. Acad. Sci. USA82: 6129–6132.
Russell, S.D. 1992. Double fertilization. Int. Rev. Cytol.140: 357–388.
Russell, S.D., Cresti, M. andDumas, C. 1990. Recent progress on sperm characterization in flowering plants. Phys. Plant.80: 669–676.
Smirnova, E.A. andBajer, A.S. 1992. Spindle poles in higher plant mitosis. Cell Motility Cytoskeleton23: 1–7.
Smirnova, E.A. andBajer, A.S. 1994. Microtubule converging centers and reorganization of the interphase cytoskeleton and the mitotic spindle in higher plantHaemanthus. Cell Motility Cytoskeleton27: 219–233.
Stoppin, V., Vantard, M., Schmit, A.-C. andLambert, A.-M. 1994. Isolated plant nuclei nucleate microtubule assembly: the nuclear surface in higher plants has centrosome-like activity. Plant Cell6: 1099–1106.
Tanaka, I., Nakamura, S. andMiki-Hirosige, H. 1989. Structural features of isolated generative cells and their protoplasts from pollen of some liliaceous plants. Gamete Res.24: 361–374.
Taylor, P., Kenrick, J., Li, Y., Kaul, V., Gunning, B.E.S. andKnox, R.B. 1989. The male germ unit inRhododendron: quantitative cytology, three-dimensional reconstruction, isolation and detection using fluorescent probes. Sex. Plant Reprod.2: 254–264.
Theunis, C.H., Pierson, E.S. andCresti, M. 1992. The microtubule cytoskeleton and the rounding of isolated generative cells ofNicotiana tabacum. Sex. Plant Reprod.5: 64–71.
Wilms, H.J. 1986. Dimorphic sperm cells in the pollen grain ofSpinacia.In M. Cresti and R. Dallai, eds., Biology of Reproduction and Cell Motility in Plants and Animals. University of Siena, Siena. pp. 193–198.
Zee, S.Y. andAziz-Un-Nisa. 1991. Mitosis and microtubules organizational changes in isolated generative cells ofAllemanda neriifolia. Sex. Plant Reprod.4: 132–137.
Zhang, H.Q. andCroes, A.F. 1981. A new medium for pollen germinationin vitro. Acta Bot. Neerl.31: 113–119.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, HQ., Bohdanowicz, J., Pierson, E.S. et al. Microtubular organization during asymmetrical division of the generative cell inGagea lutea . J. Plant Res. 108, 269–276 (1995). https://doi.org/10.1007/BF02344352
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02344352