Abstract
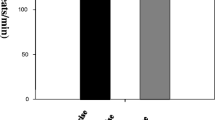
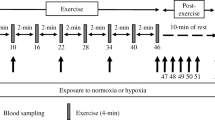
Neuroendrocrine and substrate responses were investigated in eight male athletes during inhalation of either 100% O2 (HE), 14% O2 (HO) or normoxic gas (NO) before, during and after 60 min of cycle ergometry at the same absolute work rate. Concentrations of prolactin (PRL), growth hormone (GH), testosterone (T), adrenocorticotropic hormone (ACTH), cortisol (COR), adrenalin (A), noradrenalin (NA), insulin (INS), ammonia (NH3), free fatty acids, serotonin (5-HT), total protein, branched-chain amino acids (BCAA) and free tryptophan (free TRP) were determined in venous blood and lactate concentration [LA−], partial pressure of oxygen (PO2), oxygen saturation (SO2), partial pressure of carbon dioxide and pH in capillary blood. ThePO2 andSO2 were augmented in HE and decreased in HO (P ≤ 0.01). In HO and NO no significant changes were found for any other parameter during 30 min of rest prior to exercise. In HE, PRL increased by about 400% during this time, while NA declined (P ≤ 0.01). Heart rate (HR) and [LA−] were higher during exercise in HO (P ≤ 0.01). In all trials, NH3, NA, A, T, GH and ACTH increased during exercise (P ≤ 0.01), while BCAA and INS declined. In comparison to NO and HE, increases of NA, A, GH, COR and ACTH were higher in HO (P < 0.01). The PRL in NO and COR in NO and HE did not change significantly. In HE, after the initial increase at rest, PRL declined during exercise but remained higher than in HO. Higher values for NA, A, GH, COR and ACTH in HO were likely to have reflected an augmented relative exercise intensity. Our results showed that PRL but no other hormone increased during acute exposure to hyperoxia. This PRL release was independent of exercise stress and greater than PRL augmentation during hypoxia, which was related to a higher relative exercise intensity as indicated by [LA−] and HR. Responses of plasma NH3, BCAA, free TRP and 5-HT could not explain PRL augmentation induced by the increment in bloodSO2 during hyperoxia.
Similar content being viewed by others
References
Banister EW, Cameron BJC (1990) Exercise-induced hyperammonemia: peripheral and central effects. Int J Sports Med 11 [Suppl 2]:129–142
Benker G, Jaspers C, Häusler G, Reinwein D (1990) Control of prolactin secretion. Klin Wochenschr 68:1157–1167
Boadle-Biber MC (1993) Regulation of serotonin synthesis. Prog Biophys Mol Biol 60:1–15
Borg G (1962) The reliability and validity of a physical work test. Acta Physiol Scand 55:33–36
Bouissou P, Brisson GR, Péronnet F, Héelie R, Ledoux M (1987) Inhibition of exercise-induced blood prolactin response by acute hypoxia. Can J Sport Sci 12:I 49–50
Cardelli-Cangiano P, Cangiano C, James HJ, Ceci F, Fischer JE, Strom R (1984) Effect of ammonia on amino acid uptake by brain microvessels. J Biol Chem 259:5295
Charney DS, Heninger GR, Reinhard JF, Sternberg DE, Hafstead KM (1982) The effect of intravenous L-tryptophan on prolactin and growth hormone and mood in healthy subjects. Psychopharmacology 77:217–222
Christensen NJ, Galbo H (1983) Sympathetic nervous activity during exercise. Ann Rev Physiol 45:139–153
Clemens JA, Roush ME, Fuller RW (1978) Evidence that serotonin neurons stimulate secretion of prolactin releasing factor. Life Sci 22:489
Davis JN, Carlson A, MacMillan V, Siesjö BK (1973) Brain tryptophan hydroxylation: dependence on arterial oxygen tension. Science 182:72–74
De Meirleir KL, Baeyens L, L'Hermite-Baleriaux M, L'Hermite M, Hollmann W (1985a) Exercise-induced prolactin release is related to anaerobiosis. J Clin Endocrinol Metab 60:1–3
De Meirleir KL, L'Hermite-Baleriaux M, L'Hermite M, Rost R, Hollmann W (1985b) Evidence for serotoninergic control of exercise-induced prolactin secretion. Horm Metab Res 17:380–381
Diksic M, Nagahiro S, Chaly T, Sourkes T, Yamamoto YL, Feindel W (1991) Serotonin synthesis rate measured in living dog brain by positronen emissions tomography. J Neurochem 56:153–162
Djarova T, Ilkov A, Varbanova A, Nikiforova A, Mateev G (1986) Human growth hormone, cortisol, and acid-base balance changes after hyperventilation and breath-holding. Int J Sports Med 7:311–315
Engfred K, Kjaer M, Secher NH, Friedman DB, Hanel B, Nielsen OJ, Bach FW, Galbo H, Levine DB (1994) Hypoxia and traininginduced adaptation of hormonal responses to exercise in humans. Eur J Appl Physiol 68:303–309
Escourrou P, Johnson DG, Rowell LB (1984) Hypoxemia increases plasma catecholamine concentrations in exercising humans. J Appl Physiol Respir Environ Exerc Physiol 57:1507–1511
Fischer HG, Hollmann W, De Meirleir KL (1991) Exercise changes in plasma tryptophan fraction and relationship with prolactin. Int J Sports Med 12:487–489
Freeman GB, Nielsen P, Gibson GE (1986) Monoamine neurotransmitter metabolism and locomotor activity during chemical hypoxia. J Neurochem 46:733–738
Friedman PA, Kappelman AH. Kaufman S (1972) Partial purification and characterization of tryptophan hydroxylase from rabbit hind brain. J Biol Chem 247:4165–4173
Galbo H (1983) Hormonal and metabolic adaptation to exercise. Thieme, Stuttgart
Graham TE, Pedersen PK, Saltin B (1987) Muscle and blood ammonia and lactate responses to prolonged exercise with hyperoxia. J Appl Physiol 63:1457–1462
Hawkins RA, Miller AL, Nielsen RC, Veech RL (1973) The acute action of ammonia on rat brain metabolism in vivo. Biochem J 134:1001–1008
Hesse B, Kanstrup I-L, Christensen NJ, Ingemann-Hansen T, Hansen JF, Halkjaer-Kristensen J, Petersen FB (1981) Reduced norepinephrine response to dynamic exercise in human subjects during O2 breathing. J Appl Physiol 51:176–178
Hollmann W, Venrath H (1966) Das Verhalten des kardio-pulmonalen Systems und der Muskelkraft bei Belastungen unter verschiedengradigen Sauerstoffgehalt der Luft. Schweiz Z Sportmed 14:27
Huang CC, Yonetani M, Lajevardi N, Delivoria-Papadopoulos M, Wilson DF, Pastuszko A (1995) Comparison of postasphyxial resuscitation with 100% and 21% oxygen on cortical oxygen pressure and striatal dopamine metabolism in newborn piglets. J Neurochem 64:292–298
Katz IR (1980) Oxygen affinity of tyrosine and tryptophan hydroxylases in synaptosomes. J Neurochem 35:760–763
Kjaer M, Bangsbo J, Lortie G, Galbo H (1988) Hormonal response to exercise in humans: influence of hypoxia and physical training. Am J Physiol 254:R197-R203
Kraemer WJ, Hamilton AJ, Gordon SE, Trad LA, Reeves JT, Zahn DW, Cymerman A (1991) Plasma changes in beta-endorphin to acute hypobaric hypoxia and high intensity exercise. Aviat Space Environ Med 62:754–758
MacMillan V (1987) A comparison of the effects of carbon monoxide intoxication and low-oxygen gas mixtures on cerebral biogenic amine metabolism. Brain Res 408:40–46
Mans AM, Biebuyck JF, Hawkins RA (1987) Brain tryptophan abnormalities in hyperammoneamia and liver disease. In: Bender DA, Joseph MH, Kochen W, Steinhart H (eds) Progress in trytophan and serotonin research 1986. De Gruyter, Berlin, pp 207–212
Olano M, Song D, Murphy S, Wilson DF, Pastuszko A (1995) Relationships of dopamine, cortical oxygen pressure, and hydroxyl radicals in brain of newborn piglets during hypoxia and posthypoxic recovery. J Neurochem 65:1205–1212
Seals DR, Johnson DG, Fregosi RF (1991) Hyperoxia lowers sympathetic activity at rest but not during exercise in humans. Am J Physiol 260:R873-R878
Shaar CJ, Clemens JA (1974) The role of catecholamines in the release of anterior pituitary prolactin in vitro. Endocrinology 95:1202
Song D, Olano M, Wilson DF, Pastuszko A, Tammela O, Nho K, Shorr RG (1995) Comparison of the efficacy of blood and polyethylene glycol-hemoglobin in recovery of newborn piglets from hemorrhagic hypotension: effect on blood pressure, cortical oxygen, and extracellular dopamine in the brain. Transfusion 35:552–558
Strüder HK, Hollmann W, Platen P, Duperly J, Fischer HG, Weber K (1996) Alterations in plasma free tryptophan and large neutral amino acids do not affect perceived exertion and prolactin during 90 min of treadmill exercise. Int J Sports Med 17:73–79
Sutton JR (1977) Effect of acute hypoxia on the hormonal response to exercise. J Appl Physiol 42:587–592
Wilson RG, Singhal VK, Percy-Robb I, Forrest APM, Cole EN, Boyns AR, Griffiths K (1972) Response of plasma prolactin and growth hormone to insulin hypoglycaemia. Lancet 2:1283
Author information
Authors and Affiliations
Additional information
Deceased
Rights and permissions
About this article
Cite this article
Strüder, H.K., Hollmann, W., Donike, M. et al. Effect of O2 availability on neuroendocrine variables at rest and during exercise: O2 breathing increases plasma prolactin. Europ. J. Appl. Physiol. 74, 443–449 (1996). https://doi.org/10.1007/BF02337725
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02337725