Summary
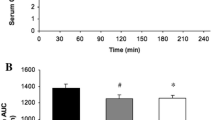
Extant literature dealing with metabolic and hormonal adaptations to exercise following carbohydrate (CHO) reduced diets is not sufficiently precise to allow researchers to partial out the effects of reduced blood glucose levels from other general effects produced by low CHO diets. In order to shed light on this issue, a study was conducted to examine the effects of a 24-h CHO-poor diet on substrate and endocrine responses during prolonged (75 min; 60%\(\dot V_{O_{2max} } \)) glucose-infused leg exercise. Eight subjects exercised on a cycle ergometer in the two following conditions: 1) after a normal diet (CHON), and 2) after a 24-h low CHO diet (CHOL). In both conditions, glucose was constantly infused intravenously (2.2 mg · kg−1 · min−1) from the 10th to the 75th min of exercise in relatively small amounts (10.4±0.8 g). No significant differences in blood glucose concentrations were found between the two conditions at rest and during exercise although a significant increase (p<0.01) in glucose level was observed in both conditions after 40 min of exercise. The CHOL as compared to the CHON condition, was associated with significantly (p<0.05) lower resting concentrations of insulin, muscle glycogen (8.7 vs 10.6 g · kg−1), and triacylglycerol, and greater concentrations of Β-hydroxybutyrate (0.5 vs 0.2 mmol · L−1), and free fatty acids. During exercise, the CHOL condition as compared to the CHON condition, was associated with significantly (p<0.05) lower insulin and R values, as well as greater free fatty acid (from min 20 to 60) and epinephrine (min 60 to 75) concentrations. Norepinephrine and glucagon concentrations also showed a net tendency (p<0.06) to be higher in the CHOL condition. There were no significant differences at rest and during exercise in blood lactate and cortisol concentrations between the two conditions. These results demonstrate that blood glucose is not the sole determinant of the metabolic and hormonal responses during prolonged exercise following a low CHO intake and indicate that other factors may be involved in the regulatory mechanism.
Similar content being viewed by others
References
Bergstrom J (1975) Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35:609–616
Bernal R, Hutson DG, Dombro RS, Levingstone A, Levi JU, Zeppa R (1982) A possible hepatic factor in the control of plasma free fatty acid levels. Metabolism 31:533–537
Bonen A, Malcolm SA, Kilgour RD, MacIntyre KP, Belcastro AN (1981) Glucose ingestion before and during intense exercise. J Appl Physiol Respirat Environ Exercise Physiol 50:766–771
Costill DL, Coyle E, Dalsky G, Evans W, Fink W, Hoopes D (1977) Effects of elevated plasma FFA and insulin on muscle glycogen usage during exercise. J Appl Physiol: Respirat Environ Exercise Physiol 43:695–699
Cousineau D, Péronnet F, Picard D, Jacks B, Ledoux M, Nadeau R, de Champlain J (1981) plasma catecholamine response to prolonged exercise after muscle glycogen depletion and overloading In: Poortmans J, Niset G (eds) Biochemistry of exercise IV-B. University Park Press, Baltimore pp 31–37
Felig P, Wahren J (1979) Role on insulin and glucagon in the regulation of hepatic glucose production during exercise. Diabetes 28: [Suppl 1] 71–75
Galbo H, Richter EA, Hilsted J (1977) Hormonal regulation during prolonged exercise. In: Milvy P (ed) The marathon physiological, medical, epidemiological and psychological studies. The New York Academy of Sciences, New York pp 72–80
Galbo H, Holst JJ, Christensen NJ (1979) The effect of different diets and of insulin on the hormonal response to prolonged exercise. Acta Physiol Scand 107:19–32
Galbo H, Christensen NJ, Mikines KJ, Sonne B, Hilsted J, Hagen C, Fahrenkrug J (1981) The effect of fasting on the hormonal response to graded exercise. J Clin Endocrinol Metab 52:1106–1112
Gollnick PD, Pernow B, Essen B, Jansson E, Saltin B (1981) Availability of glycogen and plasma FFA for substrate utilization in leg muscle of man during exercise. Clin Physiol 1:27–42
Harper HA, Rodwell VW, Mayes PA (1979) Review of physiological chemistry. Lange Medical Publications, Los Altos, ed 17, pp 299
Jansson E (1980) Diet and muscle metabolism in man. Acta Physiol Scand [Suppl] 487:1–24
Koslowski S, Brzenzinska I, Nazar K, Turlijska E (1981) Carbohydrate availability for the brain and muscles as a factor modifying sympathetic activity during exercise in dogs In: Poortmans J, Niset GF (eds) Biochemistry of Exercise IV-B, University Park Press, Baltimore, pp 54–63
Koslowski S, Nazar R, Brzezinska Z, Stephens D, Kaciuba-Uscilko H, Kobryn A (1983) Mechanism of sympathetic activation during prolonged physical exercise in dogs. Pflügers Arch 399:63–67
Lavoie J-M (1982) Blood metabolites during prolonged exercise in swimming and leg cycling. Eur J Appl Physiol 48:127–133
Lavoie J-M., Péronnet F, Cousineau D, Provencher PJ (1984) Effects of a 24-h CHO-poor diet on metabolic and hormonal responses during prolonged CHO-loaded leg exercise. Int J Sports Med 5:146–151
Lavoie J-M., Hélie R, Péronnet F, Cousineau D, Provencher PJ (1985) Effects of muscle CHO-loading manipulations on hormonal responses during prolonged exercise. Int J Sports Med 6:95–99
Lavoie J-M, Cousineau D, Péronnet F, Provencher PJ (1983) Liver glycogen store and hypoglycemia during prolonged exercise in humans. In: Knuttgen HG, Vogel JA, Poortmans J (eds) Biochemistry of exercise champaign, II, vol 13. Human Kinetics Publisher, ISSS, pp 297–301
Lo S, Russell J-C, Taylor AW (1970) Determination of glycogen in small tissue samples. J Appl Physiol 28:234–236
Maughan RJ, Williams C, Campbell DM, Hepburn D (1978) Fat and carbohydrate metabolism during low intensity exercise: effects of the availability of muscle glycogen. Eur J Appl Physiol 39:7–16
Nilsson LH Son, Hultman E (1973) Liver glycogen in man — the effect of total starvation or a carbohydrate-poor diet followed by carbohydrate refeeding. Scand. J Clin Lab Invest 32:325–330
Olsen C (1971) An enzymatic fluorimetric micromethod for the determination of acetoacetate, B-hydroxybutyrate, pyruvate and lactate. Clin Chem Acta 33:293–300
Passon PG, Peuler JD (1973) A simplified radiometric assay for plasma norepinephrine and epinephrine. Anal Biochem 51:618–631
Pequinot JM, Peyrin L, Peres G (1980) Catecholamine-fuel interrelationships during exercise in fasting men. J Appl Physiol: Respirat Environ Exercise Physiol 48:109–113
Pinelli A (1973) A new colorimetric method for plasma fatty acid analysis. Clin Chem Acta 44:385–390
Robinson AM, Williamson DH (1980) Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev 60:143–187
Russek M, Rodriguez-Zendejas AM, Pina S (1968) Hypothetical liver receptors and the anorexia caused by adrenaline and glucose. Physiol Behav 3:249–257
Russek M, Grinstein S (1974) Coding of metabolic information by hepatic glucoreceptors. In: Myers RD, Drucker-Colin RR (eds) Neurohumoral coding of brain function. Plenum Press, New York, pp 81–97
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hélie, R., Lavoie, J.M. & Cousineau, D. Effects of a 24-h carbohydrate-poor diet on metabolic and hormonal responses during prolonged glucose-infused leg exercise. Europ. J. Appl. Physiol. 54, 420–426 (1985). https://doi.org/10.1007/BF02337188
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02337188