Abstract
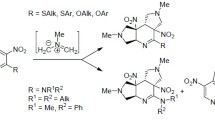
4-(3-Azatricyclo[3.2.1.0]oct-3-yl)-2,6-diazido-3,5-dicyanopyridine has been obtained by the reaction of 2,4,6-triazido-3,5-dicyanopyridine with an equimolar quantity of norbornene. The product reacted readily at room temperature with an excess of norbornene giving the corresponding trisazatricyclooctane cycloadduct. An analogous trisadduct was obtained in the reaction of 4-(3-azatricyclo[3.2.1.0]oct-3-yl)-1,6-diazido-3-chloro-5-cyanopyridine with norbornene on boiling in CCl4, and also in ether at room temperature in the presence of the complexes Rh2(OAc)4 and Cu(AcAc)2. The cycloaddition proceeds stereoselectively in all cases with the exclusive formation of exo-conformers. Calculations have been carried out using the PM3 and RHF/3-21G* methods on 2,4,6-triazido-3-chloro-5-cyanopyridine and on 2,4,6-triazido-3,5-dicyanopyridine and also on the cycloadducts of these compounds with one or two molecules of norbornene. It was established that the addition of norbornene at the azide groups of pyridine is a dipole-LUMO controlled type of reaction and leads to the formation of cycloadducts having higher LUMO energy than the initial azides. The energy of the LUMO is increased to a lesser extent as a result of the addition of norbornene to a triazide containing identical substituents in the β positions of the pyridine ring, and is due to the special features of the symmetry of the LUMO of the cycloadducts formed.
Similar content being viewed by others
References
S. V. Chapyshev and T. Ibata, Heterocycles,36, 2185 (1993).
S. V. Chapyshev, Khim. Geterotsikl. Soedin., No. 12, 1650 (1993).
S. V. Chapyshev, U. Bergstrasser, and M. Regitz, Khim. Geterotsikl. Soedin., No. 1, 67 (1996).
S. V. Chapyshev and N. V. Chapysheva, Khim. Geterotsikl. Soedin., No. 5, 666 (1994).
S. V. Chapyshev and T. Ibata, Izv. Akad. Nauk, Ser. Khim., No. 11, 2702 (1996).
S. V. Chapyshev and T. Ibata, Izv. Akad. Nauk, Ser. Khim., No. 2, 491 (1996).
E. F. V. Scriven and K. Turnbull, Chem. Rev.,88, 297 (1988).
A. Padwa and S. F. Hornbuckle, Chem. Rev.,91, 263 (1991).
H. Takeuchi, Y. Shiobara, M. Mitani, and K. Koyama, J. Chem. Soc., Chem. Commun., No. 11, 1251 (1985).
H. Takeuchi, K. Koyama, M. Mitani, R. Ihara, T. Uno, Y. Okazaki, Y. Kai, and N. Kasai, J. Chem. Soc., Perkin Trans. I, No. 3, 677 (1985).
G. L'Abbe, Chem. Rev.,69, 345 (1969).
I. R. A. Bernard, G. E. Chivers, R. J. W. Cremlyn, and K. G. Mootoosamy, Aust. J. Chem.,27, 171 (1974).
J. J. P. Stewart, J. Comput. Chem.,10, 209 (1989).
Spartan version 4.0, Wavefunction Inc., 18401 Van Karman Ave., #370 Irvine, CA 92715, USA (1995).
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. J. Su, T. L. Windus, M. Dupius, and J. A. Montgomery, J. Comput. Chem.,14, 1347 (1993).
S. V. Chapyshev and V. M. Anisimov, Khim. Geterotsikl. Soedin., No. 5, 676 (1997).
M. L. Costa, B. J. Costa Cabral, M. A. Almoster Ferreira, J. Mol. Struct.,249, 181 (1991).
A. Padwa (ed.), 1,3-Dipolar Cycloaddition Chemistry, Wiley, New York (1984), p. 559.
P. Bischof, Helv. Chim. Acta,53, 1677 (1970).
K. N. Houk, J. Sims, C. Watts, and L. Luskus, J. Am. Chem. Soc.,95, 7301 (1973).
R. Sustmann, W. Sicking, and H. Quast, J. Comput. Chem.,13, 314 (1992).
M. Tsuda, S. Oikawa, and K. Nagayama, Photogr. Sci. and Eng.,27, 118 (1983).
S. Gronowitz and P. Zanirato, J. Chem. Soc., Perkin Trans. II, No. 8, 1815 (1994).
A. Mugnoli, C. Mariani, and M. Simonetta, Acta Crystallogr.,19, 367 (1965).
Additional information
Institute of Chemical Physics in Chernogolovka, Russian Academy of Sciences, Chernogolovka 142432. Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 11, pp. 1521–1532, November, 1997.
Rights and permissions
About this article
Cite this article
Chapyshev, S.V., Anisimov, V.M. Stereo- and regioselective cycloaddition of norbornene to 2,4,9-triazidopyridine. Chem Heterocycl Compd 33, 1315–1324 (1997). https://doi.org/10.1007/BF02320334
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02320334