Zusammenfassung
Es werden verschiedene Reaktionen in der Oxindolreihe diskutiert. Man kann sie sich als ähnliche Mechanismen vorstellen, da alle über intramolekular gebildete Zwischenprodukte bzw. Endprodukte laufen.
References
R. Goutarel, M.-M. Janot, V. Prelog, andW. I. Taylor, Helv. chim. Acta33, 150 (1950).
For the best review seeP. L. Julian, E. W. Meier, andH. C. Printy, inHeterocyclic Compounds, Vol. III (John Wiley & Sons, Inc., New York, 1952), Chapter 1, pp. 161 and 225.
P. L. Julian, H. C. Printy, R. Ketcham, andR. Doone, J. Amer. Chem. Soc.75, 5305 (1953).
It is interesting to note that the cyclization of compounds containing two hetero atoms results in the formation of 5-membered lactams; e.g.:
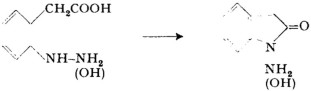 [P. W. Neber, Ber. dtsch. chem. Ges.55, 826 (1922).
[P. W. Neber, Ber. dtsch. chem. Ges.55, 826 (1922).P. W. Neber andH. Keppler, Ber. dtsch. chem. Ges.57, 778 (1924).
J. Martinet andO. Dornier, C. r. Acad. Sci.172, 1415 (1921).
F. J. Di Carlo, J. Amer. Chem. Soc.66, 1420 (1944)].
P. L. Julian, E. W. Meier, andH. C. Printy, Note 1, p. 418.
P. L. Julian, E. W. Meier, andH. C. Printy inHeterocyclic Compounds, Vol. III (John Wiley & Sons, Inc., New York, 1952), Chapter 1.
R. B. Woodward andR. H. Eastman, J. Amer. Chem. Soc.72, 399 (1950).
P. L. Julian, H. C. Printy, R. Ketcham, andR. Doone, J. Amer. Chem. Soc.75, 5305 (1953).
B. Witkop, J. Amer. Chem. Soc.72, 2311 (1950).
T. Hoshino et al., Ann. Chem.500, 42 (1932);520, 19 (1935).
P. L. Julian andJ. Pikl, J. Amer. Chem. Soc.57, 539 (1935).
R. B. Woodward andE. Wenkert, unpublished results. The authors express their thanks to ProfessorWoodward for permission to include the above data in this article.
As a sidelight to the above discussion, the interesting thermal rearrangement of 3-acylindole hydrazones should be noted [C. Alberti, Gazz. Chim. ital.77, 398 (1947)]. This reaction may be postulated to proceed via an internally interacted intermediate which breaks up into the more thermodynamically stable aminophenylpyrazole:
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wenkert, E., Reid, T.L. Intramolecular reactions in the oxindole series. Experientia 10, 417–418 (1954). https://doi.org/10.1007/BF02318503
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02318503


 [
[