Abstract
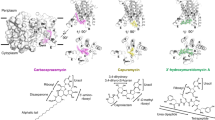
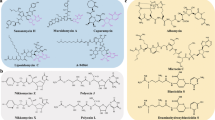
Modern synthetic approaches to DNA binding pyrrolobenzodiazepine antibiotics have been discussed.
Similar content being viewed by others
References
D. E. Thurston, in: Molecular Aspects of Anticancer Drug DNA Interaction, S. Neidle and M. J. Waring (Eds.), Macmillan, Vol. 1 (1993), pp. 54–88.
J. A. Hartley and R. L. Souhami, in: Cancer Chemotherapy; Frontiers in Pharmacology and Therapeutics, J. A. Hickman, T. Tritton (Eds.), Blackwell Scientific Ltd. (1993), pp. 251–280.
W. A. Remers, in: The Chemistry of Antitumor Antibiotics, Wiley: New York, Vol. 2 (1998), pp. 28–92.
J. A. Mountzouris, L. H. Hurley, in: Advances in DNA Sequence-Selecitive Agents, Padwa, Ed., JAI Press: London, Vol. 1 (1992), pp. 263–292.
D. E. Thurston and L. H. Hurley, Drugs Future,8, 957 (1983).
L. H. Hurely and D. E. Thurston, Pharm. Res.,2, 52 (1984).
L. H. Hurely, T. Reck, D. E. Thurston, D. R. Langley, K. G. Holden, R. P. Hertzberg, J. R. E. Hoover, G. Gallagher, Jr., L. F. Faucette, S.-M. Mong, and R. K. Johnson, Chem. Res. Toxicol.,1, 258 (1998).
L. H. Hurley and R. L. Petrusek, Nature,282, 529 (1979).
R. L. Petrusek, G. L. Anderson, T. F. Garner, Q. L. Fannin, D. J. Kaplan, S. G. Zimmer, and L. H. Hurely, Biochemistry,20, 1111 (1981).
R. L. Petrusek, E. L. Uhlenhopp, N. Duteau, and L. H. Hurley, J. Biol. Chem.,257, 6207 (1982).
R. P. Hertzberg, S. M. Hecht, V. L. Reynolds, I. J. Molineux and L. H. Hurely, Biochemistry,25, 1249 (1986).
F. L. Boyd, D. Stewart, W. A. Remers, M. D. Barkley, and L. H. Hurley, Biochemistry,32, 8712 (1993).
R. Kizu, P. H. Draves, and L. H. Hurley, Biochemistry,32, 8712 (1993).
J. A. Hartley, J. P. Bingham, and R. L. Souhani, Nucleic Acids Res.,20, 3175 (1992).
M. Lee, A. L. Rhodes, M. D. Wyatt, S. Forrow, and J. A. Hartley, Anticancer Drug Design,8, 173 (1993).
D. S. Bose, A. S. Thompson, J. Ching, J. A. Hartley, M. Berardini, T. C. Jenkins, S. Neidle, L. H. Hurley, and D. E. Thurston, J. Am. Chem. Soc.,114, 4939 (1992).
D. S. Bose, A. S. Thompson, M. Smellie, M. D. Berardini, J. A. Hartley, T. C. Jenkins, S. Neidle, and D. E. Thurston, J. Chem. Soc. Chem. Commun., No. 20, 1518 (1992).
T. C. Jenkins, S. Neidle, and D. E. Thurston, in: Chemistry of Heterocyclic Compounds, I. Stibor (Ed.), Prague Institute of Chemical Technology: Prague (1993), p. 173.
J.-J. Wan, G. C. Hill, and L. H. Hurley, J. Med. Chem.,35, 2995 (1992).
J. A. Mountzouris, J. J. Wang, D. E. Thurston, and L. H. Hurley, J. Med. Chem.,37, 3132 (1994).
W. Leimgruber, A. D. Batcho, and R. C. Czajkowski, J. Am. Chem. Soc.,90, 5641 (1968).
D. E. Thurston and D. S. Bose, Chem. Rev.,94, 433 (1994).
T. Kaneko, H. Wong, and T. W. Doyl, Tetrahedron Lett.,24, 5165 (1983).
J. W. Suggs, Y. S. Wang, and K. S. Lee, Tetrahedron Lett.,26, 4871 (1985).
J. W. Lown and A. V. Joshua, Biochem. Pharmacol.,28, 2017 (1979).
N. Langlois, F. Favre, and A. Rojas, Tetrahedron Lett.,34, 4365 (1993).
T. Kaneko, H. Wong, T. W. Doyle, W. C. Rose, and W. T. Bradner, J. Med. Chem.,28, 388 (1985).
D. R. Langley and D. E. Thurston, J. Org. Chem.,52, 91 (1987).
S. M. Courtney and D. E. Thurston, Tetrahedron Lett.,34, 5327 (1993).
D. S. Bose, G. B. Jones, and D. E. Thurston, Tetrahedron,48, 751 (1992).
M. Mori, M. Kimura, Y. Uozumi, and Y. Ban, Tetrahedron Lett.,26, 5947 (1985).
M. Mori, Y. Uozumi, and Y. Ban, J. Chem. Soc. Chem. Commun., No. 11, 841 (1986).
A. Kamal, B. S. P. Reddy, and B. S. N. Reddy, Tetrahedron Lett.,37, 2281 (1996).
M. Artico, G. De Martino, R. Giuliano, S. Massa, and G. C. Porretta, J. Chem. Soc. Chem. Commun., No. 12, 671 (1969).
M. Miyamoto, S. Kondo, H. Naganawa, K. Maeda, M. Ohno, and H. Umezawa, J. Antibiot.,50, 340 (1997).
Z. Tozuka, H. Takasugi, and T. Takaya, J. Antibiot.,36, 276 (1983).
D. E. Thurston and D. R. Langley, J. Org. Chem.,51, 705 (1986).
A. Kamal, B. S. P. Reddy, and B. S. N. Reddy, Tetrahedron Lett.,37, 2281 (1996).
D. R. Langley and D. E. Thurston, J. Org. Chem.,52, 91 (1987).
S. Eguchi, K. Yamashita, Y. Matsushita, and A. Kakehi, J. Org. Chem.,60, 4006 (1995).
P. Molina, I. Diaz, and A. Tarraga, Tetrahedron,51, 5617 (1995).
A. Kamal, N. V. Rao, and E. Laxman, Tetrahedron Lett.,38, 6945 (1997).
I. A. O'Neil, C. L. Murray, R. C. Hunter, S. B. Kalindjian, and T. C. Jenkins, Synlett., No. 1, 75 (1997).
A. Kamal, B. S. P. Reddy, and B. S. N. Reddy, Tetrahedron Lett.,37, 6803 (1996).
K. R. Prabhu, P. S. Sivanand, and S. Chandrasekaran, Synlett, No. 1, 47 (1988).
P. H. Mazzocchi and A. D. Schuda, Heterocycles,23, 1603 (1985).
D. E. Thurston, G. B. Jones, and M. E. Davies, J. Chem. Soc. Chem. Commun., No. 15, 874 (1990).
S. Mori, T. Aoyama, and T. Shion, Tetrahedron Lett.,27, 6111 (1986).
D. E. Thurston, P. T. P. Kaumaya, and L. H. Hurley, Tetrahedron Lett.,25, 4706 (1984).
J. D. Farmer, G. R. Gustafson, Jr., A. Conti, M. B. Zimmt, and J. W. Suggs, Nucleic Acids Res.,19, 899 (1991).
J. D. Farmer, S. M. Rudnick, Jr., and J. W. Suggs, Tetrahedron Lett.,29, 5105 (1988).
J. D. Farmer, Jr., Ph. D. Thesis, Brown University (1989).
I. D. Zhang and J. W. Suggs, Abstracts of Papers of American Chemical Society,204, 112 (1992).
D. S. Bose, A. S. Thompson, J. A. Ching, J. A. Hartley, M. D. Beradini, T. C. Jenkins, S. Neidle, L. H. Hurley, and D. E. Thurston, J. Am. Chem. Soc.,114, 4934 (1992).
D. E. Thurston and D. S. Bose, PCT WO 93 1845; Chem. Abstr.,120, 270468 (1994).
J. A. Mountzouris, J. J. Wang, D. E. Thurston, and L. H. Hurley, J. Med. Chem.,37, 3132 (1984).
T. C. Jenkins., L. H. Hurley, S. Neidle, and D. E. Thurston, J. Med. Chem.,37, 4529 (1994).
D. E. Thurston, D. S. Bose, A. S. Thompson, P. W. Howard, A. Leoni, S. J. Croker, T. C. Jenkins, S. Neidle, J. A. Hartley, and L. H. Hurley, J. Org. Chem.,61, 8141 (1996).
A. Kamal and N. V. Rao, Tetrahedron Lett.,36, 4299 (1995).
D. E. Thurston, S. J. Morris and J. A. Hartley, J. Chem. Soc. Chem. Commun., No. 4, 563 (1996).
S. C. Willson, P. W. Howard, and D. E. Thurston, Tetrahedron Lett.,36, 6333 (1995).
A. Kamal, Y. Damayanthi, B. S. N. Reddy, B. Lakminaryana, and B. S. P. Reddy, J. Chem. Soc. Chem. Commun., No. 11, 1015 (1997).
Additional information
Biotransformation Laboratory, Division of Organic Chemistry I, Indian Institute of Chemical Technology, Hyderabad 500007, India. Published in Khimiya Geterotsiklicheskikh Soedinenii, No. 12, pp. 1588–1604, December, 1998.
Rights and permissions
About this article
Cite this article
Kamal, A., Rao, M.V. & Satyanarayana Reddy, B. The newer synthetic strategies for DNA binding pyrrolobenzodiazepine antibiotics (review). Chem Heterocycl Compd 34, 1342–1358 (1998). https://doi.org/10.1007/BF02317805
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02317805