Abstract
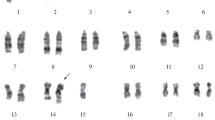
Mutants of the human melanoma cell line, FEM-X, selected in multiple steps with VP-16 (etoposide), are cross resistant to the epipodophyllotoxins and doxorubicin. Complementary DNA's for topoisomerase IIα were cloned from both FEM-X and FVP3, the most resistant mutant. Deletion of nucleotides 1320–1322 (or Ala429 from the resulting topoisomerase IIα protein) was unique to the cDNA from the drug resistant cell line. Expression of the mutant mRNA increases in parallel with VP-16 resistance in this series of cell lines. Restriction analysis and Southern analysis with allele-specific oligonucleotide probes were used to quantify the ratio of wild-type to mutant topoisomerase IIα alleles present in DNA amplified by PCR from both FEM-X and the drug resistant sublines. This analysis shows that in cell lines of increasing drug resistance, the number of mutant topoisomerase IIα alleles increases incrementally along with a concomitant decrease in the number of wild-type alleles. By quantitative Southern analysis of genomic DNA the total number of topoosomerase IIα alleles in FVP3 is approximately 2-fold that in the parental cells. Fluorescencein situ hybridization with a chromosome 17 paint reveals that amplification of the topoisomerase IIα locus in FVP3 correlates with an increase in the number of chromosome 17's, specifically the long arm. Cytogenetic analysis demonstrates that FEM-X contains three copies of chromosome 17, two of which are morphologically normal. During drug selection, FVP3 has gained 2–3 additional copies of the long arm of chromosome 17, the chromosomal location of the topoisomerase IIα locus. In this subline it is likely that three copies of the topoisomerase IIα gene are found on normal chromosome 17's and two on an isochromosome of the long arm of 17. By pulsed field gel electrophoresis, we were able to detect changes in the restriction pattern of the region of the long arm of chromosome 17 around the topoisomerase IIα locus that correlate with observed cytogenetic changes in FVP3. These results suggest that the acquisition of the mutant allele of topoisomerase IIα confers a selective advantage to cells in the presence of VP-16. As the drug concentration increased during the selection process, surviving sublines show preferential expression of the mutant topoisomerase IIα mRNA over that of the wild-type which is associated with a concomitant increase in the number of mutant topoisomerase IIα alleles.
Similar content being viewed by others
Literature Cited
Wang, J.C. (1985). DNA topoisomerases.Ann. Rev. Biochem. 54:665–697.
Osheroff, N. (1989). Biochemical basis for the interactions of type I and type II topoisomerases with DNA.Pharmac. Ther. 41:223–241.
Osheroff, N., Zechiedrich, E.L., and Gale, K.C. (1991). Catalytic function of DNA topoisomerase II.BioEssays 13:269–275.
Earnshaw, W.C., and Heck, M.M.S. (1985). Topoisomerase II is a structural component of mitotic chromosome scaffolds.J. Cell Biol. 100:1706–1715.
Goto, T., and Wang, J.C. (1984). Yeast topoisomerase II is encoded by a single-copy, essential gene.Cell 36:1073–1080.
DiNardo, S., Voelkel, K., and Sternglanz, R. (1984). DNA topoisomerase II mutant ofSaccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication.Proc. Natl. Acad. Sci. U.S.A. 81:2616–2620.
Tsai-Pflugfelder, M., Liu, L.F., Liu, A.A., Tewey, K.M., Whang-Peng, J., Knutsen, T., Huebner, K., Croce, C.M., and Wang, J. (1988). Cloning and sequencing of cDNA encoding human topoisomerase II and localization of the gene to chromosome region 17q21–22.Proc. Natl. Acad. Sci. U.S.A. 85:7177–7181.
Tan, K.B., Dorman, T.E., Falls, K.M., Chung, T.D.Y., Mirabelli, C.K., Crooke, S.T., and Mai, J.-I. (1992). Topoisomerase II alpha and topoisomerase II beta genes: characterization and mapping to human chromosomes 17 and 3, respectively.Cancer Res. 52:231–234.
Liu, L.F. (1989). DNA topoisomerase poisons as antitumor drugs.Ann. Rev. Biochem. 58:351–375.
Beck, W.T. (1989). Unknotting the complexities of multidrug resistance: The involvement of DNA topoisomerases in drug action and resistance.J. Natl. Cancer Inst. 81: 1683–1685.
Bugg, B.Y., Danks, M.K., Beck, W.K., and Suttle, D.P. (1991). Expression of a mutant DNA topoisomerase II in CCRF-CEM human leukemic cells selected for resistance to teniposide.Proc. Natl. Acad. Sci. U.S.A. 88:7654–7658.
Campain, J.A., Gottesman, M.M., and Pastan, I. (1994). A novel mutant topoisomerase II-alpha present in VP-16 resistant human melanoma cell lines has a deletion of Alanine 429.Biochemistry 33:11327–11332.
Chan, V.T.W., Ng, S.-W., Eder, J.P., and Schnipper, L.E. (1993). Molecular cloning and identification of a point mutation in the topoisomerase II cDNA from an etoposide resistant Chinese hamster ovary cell line.J. Biol. Chem. 268:2160–2165.
Cole, S.P., Chanda, E.R., Dicke, F.P., Gerlach, J.H., and Mirski, S.E.L. (1991). Non-P-glycoprotein-mediated multidrug resistance in a small cell lung cancer cell line: evidence for decreased susceptibility to drug induced DNA damage and reduced levels of topoisomerase II.Cancer Res. 51:3345–3352.
Hinds, M., Deisseroth, K., Mayes, J., Altschuler, E., Jansen, R., Ledley, F., and Zwelling, L.A. (1991). Identification of a point mutation in the topoisomerase II gene from a human leukemia cell line containing an amsacrine-resistant form of topoisomerase II.Cancer Res. 51:4729–4731.
Lee, M.-S., Wang, J.C., and Beran, M. (1992). Two independent amsacrine-resistant human myeloid leukemia cell lines share an identical point mutation in the 170 kDa form of human topoisomerase II.J. Mol. Biol. 223:837–843.
Webb, C.D., Latham, M.D., Lock, R.B., and Sullivan, D.M. (1991). Attenuated topoisomerase II content directly correlates with a low level of drug resistance in a Chinese hamster ovary cell line.Cancer Res. 51:6543–6549.
Jannatipour, M., Liu, Y.-X., and Nitiss, J.L. (1993). The top2-5 mutant of yeast topoisomerase II encodes an enzyme resistant to etoposide and amsacrine.J. Biol. Chem. 268:18586–18592.
Wasserman, R.A., Austin, C.A., Fisher, L.M., and Wang, J.C. (1993). Use of yeast in the study of anticancer drugs targeting DNA topoisomerases: expression of a functional recombinant human DNA topoisomerase II alpha in yeast.Cancer Res. 53:3591–3596.
Campain, J.A., Padmanabhan, R. Hwang, J., Gottesman, M.M., and Pastan, I. (1993). Characterization of anusual mutant of human melanoma cells resistant to anticancer drugs that inhibit topoisomerase II.J. Cell. Physiol. 155:414–425.
Miller, S.A., Dykes, D.D., and Polesky, H.F. (1988). A simple salting out procedure for extracting DNA from human nucleated cells.Nucleic Acids Res. 16:1215.
Litt, M., Kondoleon, S., Vissing, H., van Tuinen, P., and Ledbetter, D.H. (1988). Cosmid 128 defines three RFLP's on chromosome 17q23-qter.Nucleic Acids Res. 16:6251.
ISCN (1991). Guidelines for Cancer Cytogenetics, Supplement to an International System for Human Cytogenetics Nomenclature. Basel: S. Karger AG, 1991.
Smith, C.L., Lawrence, S.K., Gillespie, G.A., Cantor, C.R., Weissman, S.M., and Collins, F.S. (1987). Strategies for mapping and cloning macroregions of mammalian genomes.Methods Enzymol. 151:461–489.
Schoenlein, P.V., Shen, D.-W., Barrett, J.B., Pastan, I., and Gottesman, M.M. (1992). Double minute chromosomes carrying the human multidrug resistance 1 and 2 genes are generated from the dimerization of submicroscopic circular DNAs in colchicine-selected KB carcinoma cells.Mol. Biol. of the Cell 3:507–520.
Schoenlein, P.V., VanDevanter, D.R., and Gottesman, M.M. (1993). Extrachromosomal elements in mammalian cells. InMethods in Molecular Genetics: Vol. 2. Gene and Chromosome Analysis Part B, (ed.) Adolph, K.W., pp. 78–104.
Ueda, K., Cardarelli, C., Gottesman, M.M., and Pastan, I. (1987). Expression of a full-length cDNA for the human “MDR” gene confers resistance to colchicine, doxorubicin, and vinblastine.Proc. Natl. Acad. Sci. U.S.A. 84:3004–3008.
Eder, J.P. Jr., Chan, V.T., Niemierko, E., Teicher, B.A., and Schnipper, L.E. (1993). Conditional expression of wild-type topoisomerase II complements a mutant enzyme in mammalian cells.J. Biol. Chem. 269:13844–13849.
Charron, M., and Hancock, R. (1991). Chromosome recombination and defective genome segregation induced in Chinese hamster cells by the topoisomerase II inhibitor VM-26.Chromosoma 100:97–102.
Downes, C.S., Mullinger, A.M., and Johnson, R.T. (1991). Inhibitors of DNA topoisomerase II prevent chromatid separation in mammalian cells but do not prevent exit from mitosis.Proc. Natl. Acad. Sci. U.S.A. 88:8895–8899.
Holm, C., Stearns, T., and Bostein, D. (1989). DNA topoisomerase II must act at mitosis to prevent nondisjunction and chromosome breakage.Mol. Cell. Biol. 9:159–167.
Heiter, P., Mann, C., Snyder, M., and Davis, R.W. (1985). Mitotic stability of yeast chromosomes: a colony color assay that measures nondisjunction and chromosome loss.Cell 40:381–392.
Keith, W.N., Douglas, F., Wishart, G.C., McCallum, H.M. George, W.D., Kaye, S.B., and Brown, R. (1993). Co-amplification of erbB2, topoisomerase II alpha and retenoic acid receptor alpha genes in breast cancer and allelic loss at topoisomerase I on chromosome 20.Eur. J. Cancer 29A:1469–1475.
Smith, K., Houlbrook, S., Greenall, M., Carmichael, J., and Harris, A.L. (1993). Topoisomerase II alpha co-amplification with erbB2 in human primary breast cancer and breast cancer cell lines: relationship to m-AMSA and mitoxantrone sensitivity.Oncogene 8:933–938.
Keith, W.N., Tan, K.B., and Brown, R. (1992). Amplification of the topoisomerase IIα gene in a non-small cell lung cancer cell line and characterization of polymorphisms at the human topoisomerase IIα and B loci in normal tissues.Genes, Chrom. Cancer 4:169–175.
Schoenlein, P.V. (1994). Role of gene amplification in drug resistance. InAnticancer Drug Resistance: Advances in Molecular and Clinical Research (eds.) Goldstein, L.J., and Ozols, R.F., (Kluwer Academic Publishers) pp. 167–200.
Counillon, L., Franchi, A., and Pouyssegur, J. (1993). A point mutation of the Na+/H+ exchanger gene (NEH1) and amplification of the mutated allele confer amiloride resistance upon chronic acidosis.Proc. Natl. Acad. Sci. U.S.A. 90:4508–4512.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Campain, J.A., Slovak, M.L., Schoenlein, P.V. et al. Acquisition of multiple copies of a mutant topoisomerase IIα allele by chromosome 17 aneuploidy is associated with etoposide resistance in human melanoma cell lines. Somat Cell Mol Genet 21, 451–471 (1995). https://doi.org/10.1007/BF02310211
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02310211