Abstract
Background: The inadequacy of systemic treatments of advanced colorectal cancer has aroused interest in biologic therapy. Recent animal models have demonstrated the efficacy and safety of a recombinant vaccine that contains vaccinia and the gene for carcinoembryonic antigen (rV-CEA).
Methods: A phase I clinical trial of rV-CEA was conducted to assess vaccine toxicities, the maximum tolerated dosage, resulting immune activities, and tumor responses. A dose-escalation protocol was devised for three concentrations. Six patients per dosage were each to receive three vaccinations.
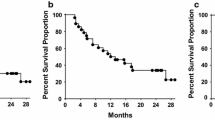
Results: Seventeen patients with advanced colorectal cancer received a total of 44 vaccinations. Mild local and systemic reactions—comparable to those seen with vaccinia alone—were observed and were typically associated with the first vaccination. No significant complications or deaths were caused by the rV-CEA. In particular, no autoimmune colitis developed, nor did leukopenia occur, despite some homology between CEA and leukocyte antigens. All three vaccine concentrations were equally well tolerated. Most patients demonstrated tumor progression by clinical and radiographic parameters and by CEA levels. Immune assays are pending.
Conclusions: This phase I trial demonstrated the safety of rV-CEA in patients with advanced colorectal cancer. Future clinical studies are warranted and will likely be influenced by investigations of the immune responses to the vaccine.
Similar content being viewed by others
References
Wingo PA, Tong T, Bolden S. Cancer statistics, 1995.CA Cancer J Clin 1995;45:8–30.
Kaufman H, Schlom J, Kantor J. A recombinant vaccinia virus expressing human carcinoembryonic antigen (CEA).Int J Cancer 1991;48:900–7.
Oikawa S, Nakazato H, Kosaki G. Primary structure of human carcinoembryonic antigen (CEA) deduced from cDNA sequence.Biochem Biophys Res Commun 1987;142:511–18.
Kantor J, Irvine K, Abrams S, Kaufman H, DiPietro J, Schlom J. Antitumor activity and immune responses induced by a recombinant carcinoembryonic antigen-vaccinia virus vaccine.J Natl Cancer Inst 1992;84:1084–91.
Kantor J, Irvine K, Abrams S, et al. Immunogenicity and safety of a vaccinia virus vaccine expressing the carcinoembryonic antigen gene in a nonhuman primate.Cancer Res 1992;52:6917–25.
Audette M, Buchegger F, Schreyer, Mach P. Monoclonal antibody against carcinoembryonic antigen (CEA) identifies two new forms of cross-reacting antigens of molecular weight 90,000 and 160,000 in normal granulocytes.Mol Immunol 1987;24:1177–86.
Harkness JW, Sands JJ, Richards MS. Serological studies of mucosal disease virus in England and Wales.Res Vet Sci 1978;24:98–103.
Hoover HC, Surdyke MG, Dangel RB, Peters LC, Hanna MG. Prospectively randomized trial of adjuvant active-specific immunotherapy for human colorectal cancer.Cancer 1985;55:1236–43.
Hollinshead A, Elias EG, Arlen M, Buda B, Mosley M, Scherrer J. Specific active immnotherapy in patients with adenocarcinoma of the colon utilizing tumor-associated antigens (TAA): a Phase I clinical trial.Cancer 1985;56:480–9.
Moss B, Flexner C. Vaccinia virus expression vectors.Annu Rev Immunol 1987;5:305–24.
Mackett M, Smith GL, Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes.J Virol 1984;49:857–64.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McAneny, D., Ryan, C.A., Beazley, R.M. et al. Results of a phase I trial of a recombinant vaccinia virus that expresses carcinoembryonic antigen in patients with advanced colorectal cancer. Annals of Surgical Oncology 3, 495–500 (1996). https://doi.org/10.1007/BF02305769
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02305769