Abstract
Background: Sucrase-isomaltase (SI) is a tissue-based phenotypic marker that is an independent prognostic factor in colorectal cancer (CRC). DF3 and galectin 3 are two other tissue-based markers that are upregulated during neoplastic transformation. Because p53 mutations are acquired during neoplastic progression, we reasoned that alterations in SI and p53 may be associated despite an apparent lack of biological interaction.
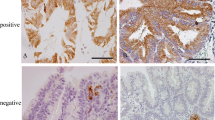
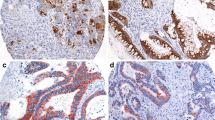
Methods: Paraffin sections from 183 patients who underwent surgery at New England Deaconess Hospital (NEDH) between 1965 and 1977 were analyzed first by immunohistochemistry (IHC) for the expression of the markers SI, DF3, and galectin 3, which were scored as absent or present. Paraffin sections from a second group of 59 patients who underwent surgery at NEDH between 1985 and 1992 were analyzed by IHC for the expression of p53 as well as SI, DF3, and galectin 3. p53 nuclear staining was scored as absent or present. Previous work has shown that p53 is mutated in all cells with nuclear staining and in 10% of tumors that are unstained.
Results: SI expression was not associated with the expression of either DF3 or galectin 3, and neither DF3 nor galectin 3 were prognostic factors in CRC. None of the phenotypic markers were associated with any of the clinicopathologic variables. However, 21 of 24 p53-positive cases (88%) expressed SI, whereas 15 of 35 p53-negative cases (43%) were also SI negative (p=0.02, Fisher exact test). p53 expression was not associated with expression of DF3 or galectin 3.
Conclusions: SI expression and p53 mutation are associated significantly in CRC. Although the mechanism underlying such an association is presently unknown, the association may define a subset of patients with a worse prognosis.
Similar content being viewed by others
References
Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis.Cell 1990;61:759–67.
Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development.N Engl J Med 1988;319:525–32.
Jen J, Kim H, Piantadosi S, et al. Allelic loss of chromosome 18q and prognosis in colorectal cancer.N Engl J Med 1994;331:213–21.
Hamelin R, Laurent-Puig P, Olschwang S, et al. Association of p53 mutation with short survival in colorectal cancer.Gastroenterology 1994;106:42–8.
Auvinen A, Isola J, Visakorpi T, Koivula T, Virtanen S, Hakama M. Overexpression of p53 and long-term survival in colon carcinoma.Br J Cancer 1994;70:293–96.
Tanaka M, Omura K, Watanabe Y, Oda Y, Nakanishi I. Prognostic factors of colorectal cancer: K-ras mutation, overexpression of the p53 protein and cell proliferative activity.J Surg Oncol 1994;57:57–64.
Zeng ZS, Sarkis AS, Zhang ZF, et al. p53 nuclear over-expression: an independent predictor of survival in lymph node-positive colorectal cancer patients.J Clin Oncol 1994;12:2043–50.
Yamaguchi A, Kurosaka Y, Fushida S, et al. Expression of p53 protein in colorectal cancer and its relationship to short-term prognosis.Cancer 1992;70:2778–84.
Bell SM, Scott N, Cross D, et al. Prognostic value of p53 overexpression and c-Ki-ras gene mutations in colorectal cancer.Gastroenterology 1993;104:57–64.
Grewal H, Guillem JG, Klimstra DS, Cohen AM. p53 nuclear overexpression may not be an independent prognostic marker in early colorectal cancer.Dis Colon Rectum 1995;38:1176–81.
O'Connell MJ, Schaid DJ, Ganju V, Cunningham J, Kovach JS, Thibodeau SN. Current status of adjuvant chemotherapy for colorectal cancer. Can molecular markers play a role in predicting prognosis?Cancer 1992;70:1732–9.
Scott N, Sagar P, Stewart J, Blair GE, Dixon MF, Quirke P. p53 in colorectal cancer: clinicopathological correlation and prognostic significance.Br J Cancer 1991;63:317–9.
Wiltz O, O'Hara CJ, Steele GD, Mercurio AM. Sucrase-isomaltase: a marker associated with the progression of adenomatous polyps of colon adenocarcinomas.Surgery 1990;108:269–75.
Wiltz O, O'Hara CJ, Steele GD, Mercurio AM. Expression of enzymatically active sucrase-isomaltase is a ubiquitous property of colon adenocarcinoma.Gastroenterology 1991;100:1266–78.
Lee EC, Woo HJ, Korzelius CA, Steele GD, Mercurio AM. Carbohydrate-binding protein 35 is the major cell-surface laminin-binding protein in colon carcinoma.Arch Surg 1991;126:1498–502.
Lotz MM, Andrews CW Jr, Korzelius CA, et al. Decreased expression of Mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma.Proc Natl Acad Sci U S A 1993;90:3466–70.
Perey L, Hayes DF, Maimonis P, Abe M, O Hara C, Kufe DW. Tumor selective reactivity of a monoclonal antibody prepared against a recombinant peptide derived from DF3 human breast carcinoma associated antigen.Cancer Res 1992;52:2563–68.
Andrews CW Jr, Jessup JM, Goldman H, et al. Localization of tumor-associated glycoprotein DF3 in normal, inflammatory, and neoplastic lesions of the colon.Cancer 1993;72:3185–90.
Jessup JM, Lavin PT, Andrews CW Jr, et al. Sucrase-isomaltase is an independent prognostic marker for colorectal carcinoma.Dis Colon Rectum 1995;38:1257–64.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations.J Am Stat Assoc 1958;53:457–81.
Gehan E. A generalized Wilcoxon test for comparing arbitrarily singly-censored data.Biometrika 1965;52:203–23.
Cox DR. Regressions models and life tables (with discussion).J R Stat Soc B 1972;34:187–220.
Jonckheere AR. A distribution-free K-sample test against ordered alternatives.Biometrika 1954;41:133–45.
Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis.J Am Stat Assoc 1952;47:583–621.
Armitage P, Berrey G.Statistical methods in medical research. Oxford, England: Blackwell, 1994:125–32.
Fisher RA.Statistical methods for research workers. 12th ed. New York: Hafner, 1954:96–97.
Kastrinakis WV, Ramchurren N, Rieger KM, Hess DT, Loda M, Steele G, Summerhayes IC. Increased incidence of p53 mutation is associated with hepatic metastasis in colorectal neoplastic progression.Oncogene 1995;11:647–52.
Traber PG. Differentiation of intestinal epithelial cells: lessons from the study of intestine-specific gene expression.Lab Clin Med 1994;123:467–77.
Jessup JM, Steele G Jr, Thomas P, et al. Molecular biology of neoplasatic transformantion of the large bowel.Surg Oncol Clin North Am 1994;3:449–77.
Darmoul D, Baricault L, Sapin C, Chantret I, Trugnan G, Rousset M. Decrease of mRNA levels and biosynthesis on sucrase-isomaltase but not dipeptidylpeptidase IV in forskolin or monensin-treated Caco-2 cells.Experientia 1991;47:1211–5.
Chantret I, Rodolosse A, Barbat A, et al. Differential expression of sucrase-isomaltase in clones isolated from early and late passages of the cell line Caco-2: evidence for glucose-dependent negative regulation.J Cell Sci 1994;107:213–25.
Wu GD, Beer DG, Moore JH, Orringer MB, Appelman HD, Traber PG. Sucrase-isomaltase gene expression in Barrett's esophagus and adenocarcinoma.Gastroenterology 1993;105:837–44.
Nikulasson S, Andrews CW Jr, Goldman H, et al. Sucrase-isomaltase expression in dysplasia associated with Barrett's esophagus and chronic gastritis.Int J Surg Pathol 1995;2:281–6.
Andrews CW Jr, O'Hara CJ, Goldman H, Mercurio AM, Silverman ML, Steele GD Jr. Sucrase-isomaltase expression in chronic ulcerative colitis and dysplasia.Hum Pathol 1992;23:774–9.
Ohannesian DW, Lotan D, Thomas P, et al. Carcinoembryonic antigen and other glycoconjugates act as ligands for galectin-3 in human colon carcinoma cells.Cancer Res 1995;55:2191–9.
Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, function as an intercellular adhesion molecule.Cell 1989;57:327–34.
Jessup JM, Kim JC, Thomas P, et al. Adhesion to carcinoembryonic antigen by human colorectal carcinoma cells involves at least two epitopes.Int J Cancer 1993;55:262–8.
Jessup JM, Petrick AT, Toth CA, et al. Carcinoembryonic antigen: enhancement of liver colonisation through retention of human colorectal carcinoma cells.Br J Cancer 1993;67:464–70.
Irimura T, Matsushita Y, Sutton RC, et al. Increased content of an endogenous lactose-binding lectin in human colorectal carcinoma progressed to metastatic stages.Cancer Res 1991;51:387–93.
Raz A, Lotan R. Endogenous galactoside-binding lectin: a new class of functional tumor cell surfaces molecules related to metastasis.Cancer Metastasis Rev 1987;6:433–52.
Schoeppner HL, Raz A, Ho SB, Bresalier RS. Expression of an endogeneous galactose binding lectin correlates with neoplastic progression in the colon.Cancer 1995;75:2818–26.
Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, et al. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin.J Biol Chem 1990;265:15286–93.
Chang SK, Dohrman AF, Basbaum CB, et al. Localization of mucin (MUC2 and MUC3) messenger RNA and peptide expression in human normal intestine and colon cancer.Gastroenterology 1994;107:28–36.
El-Deiry WS, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumor suppression.Cell 1993;75:817–25.
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein cip1 is a potent inhibitor of G1 cyclin-dependent kinases.Cell 1993;75:805–16.
Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases.Nature 1993;366:701–4.
Dulic V, Kaufmann WK, Wilson SJ, et al. p53-dependent inhibition of cyclin-dependent kinase activities in human fibro-blasts during radiation-induced G1 arrest.Cell 1994;76:1013–23.
Baas IO, Mulder JWR, Offerhaus GJA, Vogelstein B, Hamilton SR. An evaluation of six antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasm.J Pathol 1994;172:5–12.
Dix B, Robbins P, Carrello S, House A, Iacopetta B. Comparison of p53 gene mutation and protein overexpression in colorectal carcinomas.Br J Cancer 1994;70:585–90.
Bertorelle R, Esposito G, Belluco C, et al. p53 gene alterations and protein accumulation in colorectal cancer.J Clin Mol Pathol 1996;49:M85–90.
Bertorelle R, Esposito G, Del Mistro A, Belluco C, Nitti D, Lise M, Chieco-Bianchi L. Association of p53 gene and protein alterations with metastases in colorectal cancer.Am J Surg Pathol 1995;19:463–71.
Deb S, Jackson CT, Subler MA, Martin DW. Modulation of cellular and viral promoters by mutant human p53 proteins found in tumor cells.J Virol 1992;66:6164–70.
Kieser A, Weich HA, Brandner G, Marmè D, Kolch W. Mutant p53 potentiates protein kinase C induction of vascular endothelial growth factor expression.Oncogene 1994;9:963–9.
Chin KV, Ueda K, Pastan I, Gottesman MM. Modulation of the activity of the promoter of the human MDR1 gene by Ras and p53.Science 1992;255:459–62.
Zastawny RL, Salvino R, Chen J, Benchimol S, Ling V. The core promoter region of the P-glycoprotein gene is sufficient to confer differential responsiveness to wild-type and mutant p53.Oncogene 1993;8:1529–35.
Wu GD, Chen L, Forslund K, Trabert PG. Hepatocyte nuclear factor 1α (HNF-1α) and HNF-1 regulate transcription via two elements in an intestine-specific promoter.J Biol Chem 1994;25:17080–5.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lise, M., Loda, M., Fiorentino, M. et al. Association between sucrase-isomaltase and p53 expression in colorectal cancer. Annals of Surgical Oncology 4, 176–183 (1997). https://doi.org/10.1007/BF02303802
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02303802