Abstract
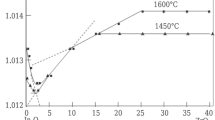
Methods of x-ray structural analysis and measurements of the mass of specimens before and after annealing and their electrophysical properties are used to investigate the formation of solid solutions based on indium oxides with additions of HfO2, CeO2, TiO2 in the range 800 – 1450 – 1600°C in air. It is established that in the system In2O3 — HfO2 annealed at 1200°C, small additions of HfO2 (0.5%) stabilize a mixture of two cubic phases. After annealing at 1450 and 1600°C a bounded region of a substitutional — interstitial — subtractive solid solution is formed. These solid solutions are preserved after successive lengthy isothermal annealings in the interval 500 – 1200°C. In the system In2O3 - CeO2 the interaction occurs at 1450°C and above with the formation of a substitutional-subtractive solid solution. Specimens containing a single-phase solid solution possess highly mobile charge carriers. All the properties of the specimens are preserved after isothermal annealing in the range 500 – 1200°C. In the system In2O3 -TiO2 with the concentration of TiO2 ranging within 0.5 – 2% a substitutional - interstitial - subtractive solid solution is formed, and for a TiO2 concentration of 2 – 5% a substitutional-subtractive solid solution is formed; above the indicated concentrations the mixture contains the compound In2TiO5 of rhombic modification. The materials of this system possess a high vaporizability.
Similar content being viewed by others
References
M. V. Varfolomeev, A. S. Mironova, and F. Kh. Chiboreva. “Interaction in the In2O3 - TiO2 system,”Izv. Akad. Nauk. SSSR, Neorganich. Materialy,11(1), 2242–2244 (1975).
L. V. Morozova, P. A. Tikhonov, and V. B. Glushkova, “Physicochemical properties of the In2O3 - HfO2 system in a region rich in indium oxide,”Izv. Akad. Nauk SSSR, Neorganich. Materialy,27(2), 291–294 (1991).
A. F. Akopov, Yu. D. Novov, and V. E. Podkletnov, “USSR Inventor's Certificate No. 1038320,”Otkrytiya, Izobreteniya, Prom. Obraztsy, Tov. Znaki, No. 32, 84 (1983).
A. E. Solov'eva and V. A. Zhdanov, “Solid solutions in the In2O3-ZrO2 system,”Izv. Akad. Nauk SSSR, Neorganich. Materialy,18(4), 840–842 (1982).
A. E. Solov'eva and V. A. Zhdanov, “Special features of the interaction of indium oxide with SnO2,”Izv. Akad. Nauk SSSR, Neorganich. Materialy,21(6), 957–960 (1985).
A. S. Okhotin,Methods for Measuring the Characteristics of Thermoelectrical Materials [in Russian], Nauka, Moscow (1974).
A. E. Solov'eva, V. A. Zhdanov, V. L. Markov, and R. R. Shvangiradze, “Properties of polycrystalline indium oxide in air and in vacuum,”Izv. Akad. Nauk. SSSR, Neorganich. Materialy,18(5), 825–828 (1982).
A. E. Solov'eva, “Investigation of structural features of CeO2-x ,”Izv. Akad. Nauk SSSR, Neorganich. Materialy,10(10), 1813–1815 (1974).
Author information
Authors and Affiliations
Additional information
Translated from Ogneupory, No. 7, pp. 21 – 23, July, 1995.
Rights and permissions
About this article
Cite this article
Solov'eva, A.E., Shvangiradze, R.R. Solid solutions based on indium oxide with additions of HfO2, CeO2, and TiO2 and some of their properties. Refractories 36, 219–222 (1995). https://doi.org/10.1007/BF02300973
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02300973