Synopsis
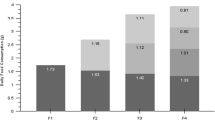
During gestation, live-bearing fishes incur physiological energy costs, including provision of energy and respiratory gases to the developing embryos and removal of waste products. Fecundity in the genusSebastes is high, and the ovaries represent a significant portion of the weight of gestating females. In this study, we compare oxygen consumption of gestating females with non-gestating females and males of kurosoi,Sebastes schlegeli, to estimate these costs. Oxygen consumption by pregnant females is significantly higher than that of males and immature females at similar sizes and weights. We estimate that a 1.5 kg gestating female consumes 68% more oxygen than a non-gestating fish during the 51.5-day period of gestation. Such an increase in oxygen consumption rates may have important implications to the metabolic scope of gestating alone, suggesting that costs of increased gill ventilation, ionic and osmotic regulation and cardiac output are relatively high. Such energetic costs represent a quantifiable expense of the viviparous mode of reproduction inSebastes as compared with oviparous species.
Similar content being viewed by others
References cited
Amoroso, E.C. 1960. Viviparity in fishes. Symp. Zool. Soc. Lond. 1: 153–181.
Balon, E.K. 1975. Reproductive guilds of fishes: a proposal and definition. J. Fish. Res. Board Can. 32: 821–864.
Barsukov, V.V. 1981. A brief review of the subfamily Sebastinae. J. Ichthyol. 21: 1–26.
Beamish, F.W.H. 1974. Apparent specific dynamic action of largemouth bass,Micropterus salmoides. J. Fish. Res. Board Can. 31: 1763–1769.
Berglund, A., G. Rosenqvist & I. Svensson. 1986. Reversed sex roles and parental energy investment in zygotes of two pipefish (Syngnathidae) species. Mar. Ecol. Prog. Ser. 29: 209–215.
Boehlert, G.W. 1978. Changes in the oxygen consumption of prejuvenile rockfish,Sebastes diploproa, prior to migration from the surface to deep water. Physiol. Zool. 51: 56–67.
Boehlert, G.W., W.H. Barss & P. Lamberson. 1982. Fecundity of the widow rockfish,Sebastes entomelas, off the coast of Oregon. U.S. Fish. Bull. 80: 881–884.
Boehlert, G.W., & R.F. Kappenman. 1980. Variation of growth with latitude in two species of rockfish (Sebastes pinniger andS. diploroa) from the northeast Pacific Ocean. Mar. Ecol. Prog. Ser. 3: 1–10.
Boehlert, G.W., M. Kusakari, M. Shimizu & J. Yamada. 1986. Energetics during embryonic development in the kurosoi,Sebastes schlegeli Hilgendorf. J. Exp. Mar. Biol. Ecol. 101: 239–256.
Boehlert, G.W. & M.M. Yoklavich. 1984. Reproduction, embryonic energetics, and the maternal-fetal relationship in the viviparous genusSebastes (Pisces: Scorpaenidae). Biol. Bull. (Woods Hole) 167: 354–370.
Burggren, W. 1985. Gas exchange, metabolism, and ‘ventilation’ in gelatinous frog egg masses. Physiol. Zool. 58: 503–514.
Calow, P. 1985. Adaptive aspects of energy allocation. pp. 13–31.In: P. Tytler & P. Calow (ed.) Fish Energetics: New Perspectives. Johns Hopkins University Press, Baltimore.
De Jager, S. & W.J. Dekkers. 1975. Relations between gill structure and activity in fish. Neth. J. Zool. 25: 276–308.
Dobbs, G.H. 1975. Scanning electron microscopy of intraovarian embryos of the viviparous teleostMicrometrus minimus (Gibbons), (Perciformes: Embiotocidae). J. Fish Biol. 7: 209–214.
Dygert, P.H. & D.R. Gunderson. 1991. Energy utilization by embryos during gestation in viviparous copper rockfish,Sebastes caurinus. Env. Biol. Fish. 30: 165–171.
Eldridge, M.B., J.A. Whipple, M.J. Bowers, B.M. Jarvis & J. Gold. 1991. Reproductive performance of yellowtail rockfish,Sebastes flavidus. Env. Biol. Fish. 30: 91–102.
Elliot, J.M. & W. Davison. 1975. Energy equivalents of oxygen consumption in animal energetics. Oecologia 19: 195–202.
Fry, F.E.J. 1971. The effect of environmental factors on the physiology of fish. pp. 1–99.In: W.S. Hoar & D.J. Randall (ed.) Fish Physiology, vol. 6, Academic Press, New York.
Gray, I.E. 1954. Comparative study of the gill area of marine fishes. Biol. Bull. (Woods Hole) 107: 219–225.
Guillemot, P.J., R.J. Larson & W.H. Lenarz, 1985. Seasonal cycles of fat and gonad volume in five species of northern California rockfish (Scorpaenidae:Sebastes). U.S. Fish. Bull. 83: 299–311.
Gunderson, D. 1971. Reproductive patterns of Pacific ocean perch (Sebastodes alutus) off Washington and British Columbia and their relation to bathymetric distribution and seasonal abundance. J. Fish. Res. Board Can. 28: 417–425.
Gunderson, D.R., P. Callahan & B. Goiney. 1980. Maturation and fecundity of four species ofSebastes. Mar. Fish. Rev. 42: 74–79.
Kusakari, M. 1978. Mariculture experiments for mass production of juvenileSebastes schlegeli. pp. 117–139.In: Annu. Rep. Hokkaido Inst. Mariculture.
Kusakari, M. 1991. Mariculture of kurosoi,Sebastes schlegeli. Env. Biol. Fish. 30: 245–251.
Larson, R.J. 1980. Influence of territoriality on adult density in two rockfishes of the genusSebastes. Mar. Biol. (Berl.) 58: 123–132.
Lenarz, W.H. & T.W. Echeverria. 1986. Comparison of visceral fat and gonadal fat volumes of yellowtail rockfish,Sebastes flavidus, during a normal year and a year of El Niño conditions. U.S. Fish. Bull. 84: 743–745.
Moser, H.G. 1967a. Reproduction and development ofSebastodes paucispinis and comparison with other rockfishes off southern California. Copeia 1967: 773–797.
Moser, H.G. 1967b. Seasonal histological changes in the gonads ofSebastodes paucispinis Ayres, an ovoviviparous teleost (Family Scorpaenidae). J. Morph. 123: 329–353.
Muir, B.S. & A.J. Niimi. 1972. Oxygen consumption of the euryhaline fish aholehole (Kuhlia sandvicensis) with reference to salinity, swimming, and food consumption. J. Fish. Res. Board Can. 29: 67–77.
Nakanishi, T. 1991. Ontogeny of the immune system inSebastiscus marmoratus: histogenesis of the lymphoid organs and effects of thymectomy. Env. Biol. Fish. 30: 135–145.
Parrish, R.H., C.S. Nelson & A. Bakun. 1981. Transport mechanisms and reproductive success of fishes in the California current. Biol. Oceanogr. 1: 175–203.
Pauly, D. 1981. The relationships between gil surface area and growth performance in fish: a generalization of von Bertalanffy's theory of growth. Meeresforsch. Rep. Mar. Res. 28: 251–282.
Priede, I.G. 1985. Metabolic scope in fishes. pp. 33–64.In: P. Tytler & P. Calow (ed.) Fish Energetics: New Perspectives, Johns Hopkins University Press, Baltimore.
Sorokin, V.P. 1961. The redfish: gametogenesis and migrations of theSebastes marinus (L.) andSebastes mentella Travin. Rapp. P.-V. Reun. Cons. Int. Explor. Mer 150: 245–250.
Trexler, J.C. 1985. Variation in the degree of viviparity in the sailfin molly,Poecilia latipinna. Copeia 1985: 999–1004.
Vahl, O. & J. Davenport. 1979. Apparent specific dynamic action of food in the fishBlennius pholis. Mar. Ecol. Prog. Ser. 1: 109–113.
Ware, D.M. 1975. Relation between egg size, growth, and natural mortality of larval fish. J. Fish. Res. Board Can. 32: 2503–2512.
Webb, P.W. & J.R. Brett. 1972. Oxygen consumption of embryos and parents, and oxygen transfer characteristics within the ovary of two species of viviparous seaperch,Rhacochilus vacca andEmbiotoca lateralis. J. Fish. Res. Board Can. 29: 1543–1553.
Wootton, R.J. 1973. The effect of size of food ration on egg production in the female three-spined stickleback,Gasterosteus aculeatus. J. Fish Biol. 5: 89–96.
Wootton, R.J. 1985. Energetics of reproduction. pp. 231–254.In: P. Tytler & P. Calow (ed.) Fish Energetics: New Perspectives, Johns Hopkins University Press, Baltimore.
Yamada, J. & M. Kusakari. 1991. Staging and the time course of embryonic development in kurosoi,Sebastes schlegeli. Env. Biol. Fish. 30: 103–110.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boehlert, G.W., Kusakari, M. & Yamada, J. Oxygen consumption of gestating femaleSebastes schlegeli: Estimating the reproductive costs of livebearing. Environ Biol Fish 30, 81–90 (1991). https://doi.org/10.1007/BF02296879
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02296879