Abstract
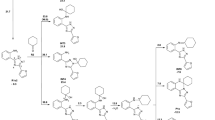
The reaction of the syn-periplanar conformer (the sp-isomer) of morpholinium 5-acetyl-6-methyl-4-(2-nitrophenyl)-3-cyano-1,4-dihydropyridine-2-thiolate with allyl bromide proceeds regio- and stereoselectively with the formation of the sp-isomer of the substituted 2-allylthio-1,4-dihydropyridine. The [3.3]-sigmatropic rearrangement of the last leads to the sp-isomer of 3,4-trans-3-allyl-5-acetyl-6-methyl-4-(2-nitrophenyl)-3-cyano-1,2,3,4-tetrahydropyridine-2(1H)-thione.
Similar content being viewed by others
References
A. M. Shestopalov, M. P. Goncharenko, V. P. Litvinov, and Yu. A. Sharanin, Dokl. Akad. Nauk,314, 1427 (1990).
V. P. Litvinov, Yu. A. Sharanin, M. P. Goncharenko, V. D. Dyachenko, and A. M. Shestopalov, Izv. Akad. Nauk, Ser. Khim, No. 8, 1997 (1991).
V. N. Nesterov, V. E. Shklover, Yu. T. Struchkov, Yu. A. Sharanin, M. P. Goncharenko, and V. D. Dyachenko, Izv. Akad. Nauk, Ser. Khim., No.2, 521 (1991).
T. P. E. auf der Heyde, H.-B. Burgi, and V. E. Shklover, Acta Crystallogr.,C47, 566 (1991).
V. N. Nesterov, V. E. Shklover, Yu. T. Struchkov, Yu. A. Sharanin, A. M. Shestopalov, and L. A. Rodinovskaya, Acta Crystallogr.,C41, 1191 (1985).
A. M. Shestopalov, O. P. Bogomolova, L. A. Rodinovskaya, V. P. Litvinov, B. Bujnicki, M. Mikolajczyk, V. N. Nesterov, and Yu. T. Struchkov, Heteroatom. Chem.,4, 593 (1993).
L. A. Rodinovskaya, A. M. Shestopalov, and V. P. Litvinov, Dokl. Akad. Nauk,330, No. 5, 597 (1993).
L. A. Rodinovskaya, Dis. Dr. Khim. Nauk, Moscow (1994).
S. Mehdi and K. Ravikumar, Acta Crystallogr.,C48, 1627 (1992).
A. Bondi, J. Phys. Chem.,70, 3006 (1966).
L. Berkovitch-Yellin and L. Leiserowitz, Acta Crystallogr.B40, 159 (1984).
G. R. Desiraju, Accounts Chem. Res.,29, 290 (1991).
F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen, and R. Taylor, J. Chem. Soc. Perkin Trans. II, 1 (1987).
Yu. A. Sharanin, A. M. Shestopalov, L. A. Rodinovskaya, V. N. Nesterov, V. E. Shklover Yu. T. Struchkov, V. K. Promonenkov, and V. P. Litvinov, Zh. Org. Khim.,22, 2600 (1986).
Yu. A. Sharanin, V. K. Promonenkov, A. M. Shestopalov, V. N. Nesterov, S. N. Melenchuk, V. E. Shklover, and Yu. T. Struchkov, Zh. Org. Khim.,25, 622 (1989).
W. Robinson and G. M. Sheldrick, Crystallographic Computing Techniques and New Technologies, Oxford Univ. Press, Oxford (1988), p. 366.
Additional information
Deceased.
A. N. Nesmeyanov Institute of Hetero-organic Compounds, Russian Academy of Sciences (RAN), Moscow 117813. N. D. Zelinskii Institute of Organic Chemistry, RAN, Moscow 117913. Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 5, pp. 643–652, May, 1997.
Rights and permissions
About this article
Cite this article
Nesterov, V.N., Rodinovskaya, L.A., Shestopalov, A.M. et al. Investigation of the stereochemistry of the [3.3]-sigmatropic rearrangement of the sp-isomer of 2-allylthio-5-acetyl-6-methyl-4-(2-nitrophenyl)-3-cyano-1,4-dihydropyridine. Chem Heterocycl Compd 33, 557–565 (1997). https://doi.org/10.1007/BF02291939
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02291939