Abstract
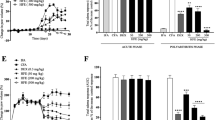
A single dose of either cyclosporin-A (CsA) or lobenzarit (CCA) given with an arthrogenic adjuvant completely prevented expression of experimental adjuvant arthritis in rats. The aim of this study was to understand how these drugs prevented the arthritis expression by studying the popliteal lymph nodes draining the arthritic joints at various times after adjuvant injection. Neither drug affected the proliferation in popliteal lymph nodes at the time arthritis was normally expressed, however, there was a marked change in the types of cells present. Immunofluorescence assays showed a reduction in the proportion of CD4+ cells, while the proportion of B-lymphocytes was almost doubled. This coincided with a marked elevation in the ability of these cells to produce interleukin (IL)-6. At the same time production of other cytokines (IL-2), tumour necrosis factor (TNF) and interferon (IFN)-γ) was not greatly affected. However, one day after adjuvant injection IL-2 and IFN-γ production was reduced. In vitro experiments showe that IL-6 production by lymphoid cells was relatively unaffected by CsA and CCA but IL-2, TNF and IFN-γ were suppressed by CsA. The results indicate that CsA and CCA may modify the response to the arthritic adjuvant by specifically inhibiting IL-2, TNF and IFN-γ production at the time of adjuvant injection. The lack of inhibition of IL-6 by these drugs reveals it may not play a key role in the initiation of this model of chronic inflammation.
Similar content being viewed by others
References
Elliott MJ, Maini RN, Feldmann M, Long-Fox A, Charles P, Katsikis P, et al. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor α. Arthr Rheum 1993;36:1681–90.
Haynes DR, Garrett IR, Vernon-Roberts B. Effect of gold salt treatment on receptor binding activity of monocytes and macrophages isolated from rats with adjuvant arthritis. Rheumatol Int 1988;8:159–64.
Connolly KM, Stecher VJ, Danis E, Purden DJ, LaBrie T. Alteration of interleukin-1 activity and the acute phase response in adjuvant arthritic rats treated with disease modifying drugs. Agents Actions 1988;25:94–105.
Dougados M, Torley H. Efficacy of cyclosporin A in rheumatoid arthritis: worldwide experience. Br J Rheumatol. 1993;(1 Suppl)32:57–9.
Sany J. Immunological treatment of rheumatoid arthritis. Clin Exp Rheumatol 1990;(5 Suppl)8:81–8.
Clerici M, Shearer GM. Differential sensitivity of human T helper cell pathways by in vitro exposure to cyclosporin A. J Immunol 1990;144:2480–5.
Katagiri K, Nakano T, Sugawara Y, Ichikawa Y, Yoshida T. CCA (disodium 4-chloro-2,2′-iminodibenzoate) inhibits the progression of human T cell proliferation triggered by PHA. Clin Immunol Immunopathol 1990;56:384–92.
Varey A, Champion BR, Cooke A, Cyclosporin affects the function of antigen-presenting cells. Immunol 1986;57;111–4.
Takeuchi T, Koide J, Hosono O, Takano M, Abe T. CCA [N-(2-carboxyphenyl)-4-chloroanthranilic acid sodium sal], a newly developed immunomodulating drug, suppresses T-cell activation by acting on macrophages. Inflammation 1989;13:124–35.
Klaus GGB. Cyclosporin-sensitive and cyclosporin-insensitive modes of B cell stimulation. Transplantation 1988;46:11S-4.
Hirohata S, Shinohara S, Inuoue T, Miyamoto T, Lipsky PE. Regulation of B cell function by lobenzarit, a novel disease-modifying antirheumatic drug. Arthr Rheum 1992;35:168–74.
Takeda Y, Urakawa K, Sakamoto A, Nakano T, Sugawara Y, Ohsugi Y, et al. Lobenzarit disodium (CCA) inhibits in vitro immunoglobulin production via direct interaction with B lymphocytes. Chem Pharm Bull Tokyo 1992;40:177–181.
Del-Pozo E, Graeber M, Elford P, Payne T. Regression of bone and cartilage loss in adjuvant arthritic rats after treatment with cyclosporin A. Arthr Rheum 1990;33:247–52.
Whitehouse MW, Rainsford KD, Taylor RM, Vernon-Roberts B. Zinc monoglycerate: A slow release source of zinc with antiarthritic activity in rats. Agents Actions 199031:47–58.
Imaizumi K, Hinoue H, Ueno M, Takata I, Sato T, Minato Y, et al. Pathological evaluation of anti-rheumatic drugs on type II collagen-induce arthritis in DBA/1J mouse. Jikken Dobutsu 1991;40:95–9.
Whitehouse MW, Vernon-Roberts B. Prevention of rat polyarthritis induced with mycobacterial or avridine adjuvants. XVIIth ILAR Congress of Rheumatology 1989;13.
Haynes DR, Garrett IR, Whitehouse MW, Vernon-Roberts B. Do gold drugs inhibit interleukin-1? Evidence from an in vitro lymphocyte activating factor assay. J Rheumatol 1988;15:775–8.
Haynes DR, Whitehouse MW, Vernon-Roberts B. The prostaglandin analogue, Misoprostol regulates inflammatory cytokines and immune functions in vitro like the natural prostaglandins E2, E2 and E3. Immunol 1992;76:251–7.
Bazin H, Xhurdebise LM, Burtonboy G, LeBaq AM, De Clercq L, Cormont F. Rat monoclonal antibodies. i. rapid purification from in vitro culture supernatants. J Immunol Meth 1984;66:261–9.
Hunig T, Wallny H-J, Hartley JK, Lawetzky A, Tiefenthaler G. A monoclonal antibody to a constant determinant of the rat T cell antigen receptor that induces T cell activation: different reactivity with subsets of immature and mature T lymphocytes. J Exp Med 1989;169:73–86.
Fukumoto T, McMaster WR, Williams AF. Mouse monoclonal antibodies against rat major histocompatibility antigens. Two Ia antigens and class I antigens in rat thymus. Eur J Immunol 1982;12:237–43.
Dallman MJ, Mason DW, Webb M. The roles of host and donor cells in the rejection of skin allografts by T cell-deprived rats injected with syngenetic T cells. Eur J Immunol 1982;12:511–18.
Mason DW, Brideau RJ, McMaster WR, Webb M, White RAH, Williams AF. Monoclonal antibodies that define T-lymphocyte subsets in the rat. In: Kennett RH, McKearn TJ, Bechtol KB, editors. Monoclonal antibodies. London: Plenum Publishing Corp., 1980;251–301.
Gadd SJ, Ashman LK. Binding of mouse monoclonal antibodies to human leukemia cells via the Fc receptor: a possible source of ‘false positive’ reactions in specificity. Clin Exp Immunol 1983;54:811–8.
Snedecor GW, Cochran WG, editors. Statistical Methods. 8th ed. Ames, Iowa: Iowa State University Press, 1989.
Madhok R, Capell HA. Cyclosporin A in Rheumatoid Arthritis: Results at 30 months. Transplant Proc 1988;20(4 Suppl):248–52.
Cohen IR, Holoshitz J, Van Eden, Frenkel A. T lymphocyte clones illuminate pathogenesis and affect therapy of experimental arthritis. Arthr Rheum 1985;28:841–5.
Larsson P, Holmdahl R, Denker L, Klareskog L. In vivo treatment with W3/13 (anti-pan T) but not with OX8 (antisuppressor/cytotoxic T) monoclonal antibodies impedes the development of adjuvant arthritis in rats. Immunol 1985;56:383–91.
Hess AD, Tutschka PJ, Santos GW. Effect of cyclosporin A on human lymphocyte responses in vitro. III. CsA inhibits the production of T lymphocyte growth factors in secondary mixed lymphocyte responses but does not inhibit the response of primed lymphocytes to TCGF. J Immunol 1982;128:355–91.
Groeniwegen G, Buurman WS, Jeanhomme GMA, Van Der Linden CJ. Effect of cyclosporin on MHC class II antigen expression on arterial and venous endothelium in vitro. Transplantation 1985;40:21–5.
Kunkl A, Klauss GGB. Selective effects of cyclosporin A on functional B cell subsets. J Immunol 1980;125:2526–31.
White DJD, Plumb A, Clane RY. The immune status of transplantation recipients immunosuppressed with cyclosporin A Transplant Proc 1980;13:1666–8.
Van Den Broek MF, Van Den Langerit LG, Van Bruggen MC, Billingham MEJ, Van den Berg WB. Treatment of rats with monoclonal anti-CD4 induces long term resistance to streptococcal cell wall-induced arthritis. Eur J Immunol 1992;22:57–61.
Mosmann TR, Coffman RL. TH1 and TH2: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989;7:145–73.
Andersson J, Nagy S, Groth C-G, Andersson U. Effects of FK 506 and cyclosporin A on cytokine production studies in vitro at a single-cell level. Immunol 1992;75:136–42.
Theisen-Popp P, Pape H, Müller-Peddinghaus R. Interleukin-6 in adjuvant arthritis of rats and its pharmacological modulation. Int J Immunopharmacol 1992;14:565–71.
Smith-Oliver T, Noel LS, Stimpson SS, Yarnall DP, Connolly KM. Elevated levels of TNF in the joints of adjuvant arthritic rats. Cytokine 1993;5:298–304.
Brauer R, Kette H, Henzgen S, Thoss K. The influence of cyclosporin A on cytokine levels in synovial fluid and serum in rats with antigen induced arthritis. Agents Actions 1994;41:96–8.
Weinblatt M, Helfgott S, Coblyn J, Spragg J, Uedelhoven WM, Tumel S, et al. The effects of cyclosporin A on eicosanoid excretion in patients with rheumatoid arthritis. Arthr Rheum 1991;34:481–5.
Hart PH, Whitty GA, Piccoli DS, Hamilton JA. Control by IFN-γ and PGE2 of TNFα and IL-1 production by human monocytes. Immunol 1989;66:374–83.
Reem GH, Cook LA, Vilcek J. Gamma interferon synthesis by human thymocytes and T lymphocytes inhibited by cyclosporin A. Science 1983;222:63–65.
Hirohata S. Regulation of in vitro anti-DNA antibody production by a novel disease modifying anti-rheumatic drug, Lobenzarit. Clin Exp Rheumatol 1992;10:357–63.
Okamoto M, Sasano M, Goto M, Nishioka K, Nakamura K, Yokohari R. Suppressive effect of anti-rheumatic drugs on interleukin-1 beta release from human peripheral blood monocytes. Int J Immunopharmacol 1991;13:39–43.
Kawakami A, Eguchi K, Ueki Y, Migita K, Ida H, Nakao H, et al. Effects of lobenzarit disodium on human endothelial cells. Lobenzarit disodium inhibits proliferative response, HLA-DR antigen expression, and T cell adherence toward endothelial cells. Arthr Rheum 1991;34:296–303.
Author information
Authors and Affiliations
Additional information
accepted by R. O. Day
Rights and permissions
About this article
Cite this article
Haynes, D.R., Gadd, S.J., Whitehouse, M.W. et al. Complete prevention of the clinical expression of adjuvant-induced arthritis in rats by cyclosporin-A and lobenzarit: The regulation of lymph node cell populations and cytokine production. Inflamm Res 45, 159–165 (1996). https://doi.org/10.1007/BF02285155
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02285155