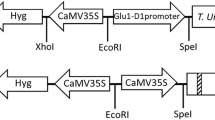
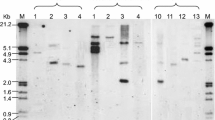
Abstract
Two different attempts have been undertaken to improve the amino acid composition of storage proteins from field bean (Vicia faba) by genetic engineering. First, legumin was modified to generate a new peptide sequence at the C-terminus containing 4 methionine residues. Second, vicilin was modified by generating 8 single methionine residues distributed over the peptide sequence. The genes were expressed in different systems includingin vitro transcription and translation and stable transformation into tobacco. The modified legumin was found to be unstable when expressed in tobacco seeds. Although specific mRNA was detected on RNA gel blots, no protein could be found by using protein gel blotting and ELISA. Furthermore, a protease preparation able to process the original legumin precursorin vitro degraded the modified legumin precursor. Contrary, the modified vicilin was accumulated in seeds of tobacco transformed with the gene under the control of the seed specific USP promoter. Both the original and the modified vicilin could be detected on protein gel blots at the expected position. Two-dimensional electrophoresis was employed to analyse the expression of original vicilin. Three vicilin-specific products of almost equal size were observed, indicating a slight modification leading to a change of pI. Quantitative determination using competitive ELISA showed that there is no significant difference in accumulation between original and modified vicilin. In both cases, three plants were found with vicilin amounts in the range of 1–3% of total globulin.
Similar content being viewed by others
References
Altenbach SB, Kuo C, Staraci LC, Pearson KW, Wainwright C, Georgescu A, Townsend J: Accumulation of a Brazil nut albumin in seeds of transgenic canola results in enhanced levels of seed protein methionine. Plant Mol Biol 18: 235–245 (1992).
Altenbach SB, Pearson KW, Meeker G, Staraci LC, Sun SM: Enhancement of the methionine content of seed proteins by the expression of a chimeric gene encoding a methionine-rich protein in transgenic plants. Plant Mol Biol 13: 513–522 (1989).
Ammerer G: Expression of genes using the ADC1 promoter. Meth Enzymol 101C: 192–201 (1983).
An G: Binary Ti vectors for plant transformation and promoter analysis. Meth Enzymol 153: 292–305 (1987).
Bassüner R, Nong Van H, Jung R, Saalbach G, Müntz K: The primary structure of the predominating vicilin storage protein subunit from field bean seeds (Vicia faba L. var.minor cv. Fribo). Nucl Acids Res 15: 9609 (1987).
Bäumlein H, Boerjan W, Nagy I, Bassüner R, Van Montagu M, Inze D, Wobus U: A novel seed protein gene fromV. faba is developmentally regulated in transgenic tobacco andArabidopsis plants. Mol Gen Genet 225: 459–467 (1991).
Bäumlein H, Müller A, Schiemann J, Helbing D, Manteuffel R, Wobus U: A legumin B gene ofVicia faba is expressed in developing seeds of transgenic tobacco. Biol Zentralbl 106: 569–575 (1987).
Bäumlein H, Wobus U, Pustell J, Kafatos FC: The legumin gene family: structure of a B type gene ofVicia faba and a possible legumin gene specific regulatory element. Nucl Acids Res 14: 2707–2720 (1986).
Botstein SY, Falco SC, Stewart SE, Brennan M, Scherer S, Stinchcomb DT, Struhl K, Davis PW: Sterile host yearst (SHY): a eucaryotic system of biological containment for recombinant DNA experiments. Gene 8: 17–24 (1979).
Bradford M: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72: 248–254 (1976).
Cremer F, van de Walle C: Method for extraction of proteins from green plant tissue for two-dimensional polyacrylamid gel electrophoresis. Anal Biochem 147: 22–26 (1985).
De Clercq A, Vandewiele M, Van Damme J, Guerche P, Van Montague M, Vandekerckhove J, Krebbers E: Stable accumulation of modified 2S albumin seed storage proteins with higher methionine contents in transgenic plants. Plant Physiol 94: 970–979 (1990).
Deblaere R, Bytebier B, DeGreeve H, Deboek F, Schell J, Van Montagu M, Leemans J: Efficient octopine Ti plasmid-derived vectors forAgrobacterium-mediated gene transfer to plants. Nucl Acids Res 13: 4777–4788 (1985).
Dickinson CD, Floener LA, Lilley GG, Nielsen NC: Self-assembly of proglycinin and hybrid proglycinin synthesized in vitro from cDNA. Proc Natl Acad Sci USA 84: 5525–5529 (1987).
Dickinson CD, Hussein EHA, Nielsen NC: Role of post-translational cleavage in glycinin assembly. Plant Cell 1: 459–469 (1989).
Dickinson CD, Scott MP, Hussein EHA, Argos P, Nielsen NC: Effect of structural modifications on the assembly of a glycinin subunit. Plant Cell 2: 402–413 (1990).
Doyle J, Schuler M, Godette WD, Zenger V, Beachy R, Slightom J: The glycosylated seed storage proteins ofGlycine max andPhaseolus vulgaris: structural homologies of genes and proteins. J Biol Chem 261: 9228–9238 (1986).
Dyer JM, Nelson JW, Murai N. Molecular modelling of methionine enhancement for the bean seed storage protein phaseolin. XV. International Botanical Congress (Yokohama, Japan), p. 554 (1993).
Gatehouse JA, Lycett GW, Croy RRD, Boulter D: The post-translational protecolysis of the subunits of vicilin from pea (Pisum sativum L.). Biochem J 207: 629–632 (1982).
Gross E: The cyanogen bromide reaction. Meth Enzymol 33: 238–255 (1967).
Hoffman LM, Donaldson DD, Herman EM: A modified storage protein is synthesized, processed and degraded in the seeds of transgenic plants. Plant Mol Biol 11: 717–730 (1988).
Horsch RB, Fry JE, Hoffman NL, Eichholtz D, Rogers SG, Fraley RT: A simple and general method for transferring genes into plants. Science 227: 1229–1231 (1985).
Horstmann C: Specific subunit pairs of legumin fromVicia faba. Phytochemistry 22: 1861–1866 (1983).
Horstmann C, Schlesier B, Otto A, Kostka S, Müntz K: Polymorphism of legumin subunits from field bean (Vicia faba L. var.minor) and its relation to the corresponding multigene family. Theor Appl Genet 86: 867–874 (1993).
Ito H, Fukada Y, Murata K, Kimura A: Transformation of intact yeast cells treated with alkali cations. J Bact 153: 163–168 (1983).
Jung R, Saalbach G, Nielsen NC, Müntz K: Site-specific limited proteolysis of legumin chloramphenicol acetyl transferase fusionsin vitro and in transgenic tobacco seeds. J Exp Bot 44 (Suppl): 343–349 (1993).
Kim C, Kamiya S, Sato T, Utsumi S, Kito M: Improvement of nutritional value and functional properties of soybean glycinin by protein engineering. Protein Engng 3: 725–731 (1990).
Ko T-P, Ng JD, McPherson A: The three-dimensional structure of canavalin from jack bean (Canavalis ensiformis). Plant Physiol 101: 729–744 (1993).
Lawrence MC, Suzuki E, Varghese JN, Davis PC, Van Donkelaar A, Tulloch PA, Colman PM: The three dimensional structure of the seed storage protein phaseolin at 3 Å resolution. EMBO J 9: 9–15 (1990).
Messing J: The manipulation of zein genes to improve the nutritional value of corn. Trends Biotechnol 1: 54–59 (1983).
Newbigin EJ, de Lumen BO, Chandler PM, Gould A, Blagrove RJ, March JF, Kortt AA, Higgins JV: Pea convicilin: structure and primary sequence of the protein and expression of a gene in the seeds of transgenic tobacco. Planta 180: 461–470 (1990).
Nielsen NC, Dickinson CD, Cho TJ, Thanh VH, Scallon BJ, Sims TL, Fischer RL, Goldberg RB: Characterization of the glycinin gene family from soybean. Plant Cell 1: 313–328 (1989).
Saalbach G, Jung R, Kunze G, Saalbach I, Adler K, Müntz K: Different legumin protein domains act as vacuolar targeting signals. Plant Cell 3: 695–708 (1991).
Saalbach G, Jung R, Saalbach I, Müntz K: Construction of storage protein genes with increased number of methionine codons and their use in transformation experiments. Biochem Physiol Pflanzen 183: 211–218 (1988).
Saalbach I, Pickardt T, Machemehl F, Saalbach G, Schieder O, Müntz K: A chimeric gene encoding the methionine-rich 2S albumin of the Brazil nut (Bertholletia excelsa HBK) is stably expressed and inherited in transgenic grain legumes. Mol Gen Genet 242: 226–236 (1994).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Sano M, Kawashima N: Isolation and partial characterization of the major seed protein fromNicotiana tabacum, and accumulation during development. Agric Biol Chem 47: 1305–1310 (1983).
Scholz G, Manteuffel R, Müntz K, Rudolph A: Low molecular weight polypeptides of vicilin fromVicia faba L. are products of proteolytic breakdown. Eur J Biochem 132: 103–107 (1983).
Scott MP, Jung R, Müntz K, Nielsen NC: A protease responsible for post-translational cleavage of a conserved Asn-Gly linkage in glycinin, the major seed storage protein of soybean. Proc Natl Acad Sci USA 89: 658–656 (1992).
Sonnewald U: Konstruktion und Analyse von chimären Patatingenen unter besonderer Berücksichtigung der Stabilität und subzellulären Kompartimentierung des Patatinproteins. Ph.D. Thesis, Free University Berlin (1989).
Southern EM: Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98: 503–517 (1975).
Spencer D, Chandler PM, Higgins TJV, Inglis AS, Rubira M: Sequence interrelationships of the subunits of vicilin from pea seeds. Plant Mol Biol 2: 259–267 (1983).
Staswick PE, Hermodson MA, Nielsen NC: The amino acid sequence of the A2B1a subunit of glycinin. J Biol Chem 259: 13424–13430 (1984).
Thomas PS: Hybridization of denatured RNA transferred or dotted to nitro-cellulose paper. Meth Enzymol 100: 255–266 (1983).
Towbin H, Staehelin T, Gordon J: Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets. Procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354 (1979).
Utsumi S, Kitagawa S, Katsube T, Kang IJ, Gidamis AB, Takaiwa F, Kito M: Synthesis, processing and accumulation of modified glycinins of soybean in the seeds, leaves and stems of transgenic tobacco. Plant Sci 92: 191–202 (1993).
Vaintraub M, Kotova L, Shaha R: Protein deamidase from germinating wheat grains. FEBS Lett 302: 169–171 (1992).
Van Haute E, Joos H, Maes M, Warren G, Van Montagu M, Schell J: Intergeneric transfer and exchange recombination of restriction fragments cloned in pBR322: a novel strategy for reversed genetics of Ti plasmids ofAgrobacterium tumefaciens. EMBO J 2: 411–417 (1983).
Vieira J, Messing J: Production of single-stranded plasmid DNA. Meth Enzymol 153: 3–11 (1987).
Wallace JC, Galili G, Kawata EE, Cuellar RE, Shotwell MA, Larkins BA: Aggregation of lysine-containing zeins into protein bodies inXenopus oocytes. Science 240: 662–664 (1988).
Weschke W, Bäumlein H, Wobus U: Nucleotide sequence of a field bean (Vicia faba L. var.minor) vicilin gene. Nucl Acids Res 15: 1065 (1987).
Wright DJ: The seed globulins. In: Hudson BJF (ed), Developments in Food Proteins 6, pp. 119–178, Elsevier, London (1988).
Zambryski P, Joos H, Genetello C, Leemans J, Van Montagu M, Schell J: Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J 2: 2143–2150 (1983).
Zoller MJ, Smith M: Oligonucleotide directed mutagenesis of DNA fragments cloned into M13 derived vectors. Meth Enzymol 190: 468–500 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Saalbach, G., Christov, V., Jung, R. et al. Stable expression of vicilin fromVicia faba with eight additional single methionine residues but failure of accumulation of legumin with an attached peptide segment in tobacco seeds. Mol Breeding 1, 245–258 (1995). https://doi.org/10.1007/BF02277425
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02277425