Abstract
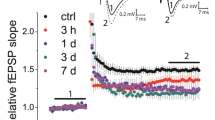
Experiments on hippocampus slices of rats showed that the pattern of induction of long-term post-tetanic potentiation of synaptic transmission is determined by the frequency of tetanic stimulation of Schaffer collaterals. With their high-frequency (>10/sec) stimulation, a phase of reversible increase in the amplitude of population EPSP (pEPSP) is observed within the initial 30-min-long interval; it is related to an increase in the intracellular Ca2+ concentration resulting from simultaneous activation of NMDA and metabotropic glutamate receptors and voltage-activated calcium channels. With the participation of calmodulin, Ca2+ activate Ca2+-calmodulin-dependent protein kinase II. The latter phosphorylates AMPA/kainate receptors (their kainate-responsive compartments), which promotes an increase in their chemosensitivity. Under conditions of low-frequency (<10/sec) tetanic stimulation of synaptic inputs, for the same reasons, an increase in the intradendritic Ca2+ concentration exerts no expressed influence on protein kinase II, but activates calcineurin. The latter, with the involvement of other phosphoprotein phosphatases, dephosphorylates AMPA/kainate receptors and turns some of them into the refractory state; this is expressed in a reversible depression of pEPSP. After 30 min of either high-frequency, or low-frequency stimulation, a non-decremental phase of long-term post-tetanic potentiation develops, which is related to the increase in the protein kinase C activity, phosphorylation of the AMPA-responsible compartments of AMPA/kainate receptors, their rising sensitivity, and a stable increase in the pEPSP amplitude
Similar content being viewed by others
References
R. C. Malenka, “Postsynaptic events mediating LTP”, in:Excitatory Amino Acids and Synaptic Transmission, H. V. Wheal and A. M. Thomson (eds.), Acad. Press, London (1991), pp. 303–314.
P. Rossi and D. Angelo, “Synaptic mechanisms of long-term potentiation”Funct. Neurol.,7, No. 1, 57–70 (1992).
R. Malinov, “LIP: desperately seeking resolution”,Science,266, 1195–1196 (1994).
Yi Li, Xu Shi-Tong, and Qu Ying-Qi, “The learning dependent on long-term synaptic potentiation in areaCA3 of hippocampus of rat,”Acta Physiol. Sin.,41, No. 3, 223–230 (1989).
O. A. Ramirez, R. A. Gomez, and H. T. Carrer, “Differences in hippocampal synaptic plasticity in rats with inborn or low learning ability may be related to different sensitivity of aspartate receptors,”Brain Res. Bull.,27, No. 2, 291–293 (1991).
G. Tocco, A. J. Annala, M. Baudry, et al. “Learning of hippocampal-dependent conditioning task changes the binding properties of AMPA receptors in rabbit hippocampus,”Behav. Neural. Biol.,58, No. 3, 222–231 (1992).
T. V. P. Bliss, M. L. Errington, M. A. Linch, et al., “Presynaptic mechanisms in hippocampal long-term potentiation,”Cold Spring Harbor Symp. Quant. Biol.,55, 119–129 (1990).
L. L. Voronin, U. Kuhut, and A. G. Gusev, “Changes in quantal parameters of CA1 synaptic transmission are dependent on the magnitude of long-term potentiation,”Neurosci. Res. Commun.,9, No. 1, 1–7 (1991).
L. L. Voronin, A. G. Gusev, N. V. Ivanov, et al., “Evaluation of the quantum content in excitatory synapses of theCA1 hippocampal region before and after tetanization: use of a deconvolution technique,”Neirofiziologiya/Neurophysiology,25, No. 1, 84–91 (1993).
A. M. Thomson and S. Radpour, “Properties of synapses mediated by excitatory amino acid and their involvement in synaptic plasticity,” in:Excitatory Amino Acids and Synaptic Transmission, H. V. Wheal and A. M. Thomson (eds.), Acad. Press, London (1991), pp. 316–332.
T. Manabe, O. J. Manzoni, and R. A. Nicol, “Long-term potentiation: evidence against an increase in transmitter release probability in theCA1 region of the hippocampus,”Science,265, No. 5182, 1880–1882 (1994).
G. L. Collingridge, S. J. Kehl, and H. McLennan, “Excitatory amino acids in synaptic transmission in the Schaffer collateral — pathway of the rat hippocampus,”J. Physiol,343, 33–46 (1983).
G. Lynch, J. Larson, S. Kelso, et al., “Intracellular injections of EGTA block induction of hippocampal long-term poterm potentiation,”Nature,365, No. 5936, 719–721 (1983).
J. A. Kauer, R. C. Malenka, and R. A. Nicoll, “NMDA application potentiates synaptic transmission in the hippocampus,”Nature,334, No. 6188, 250–252 (1988).
F. Aszetely, E. Hanse, H. Wigstrom, et al., “Synaptic potentiation in the hippocampalCA1 region induced by application of N-methyl-D-aspartate,”Brain Res.,558 No. 1/2, 153–156 (1991).
R. Dingledine, “N-methyl-D-aspartate activates voltage-dependent calcium conductance in rat hippocampal pyramidal cells,”J. Physiol,343, 385–405 (1983).
R. E. Westenbroek, M. K. Ahlijanian, and W. A. Catterall, “Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons,”Nature,347, No. 6290, 281–283 (1990).
S. Oh and B. P. Melaslin, “The iron component of sodium nitroprusside blocks NMDA-induced glutamate accumulation and intracellular Ca2+ elevation,”Neurochem. Res.,20, No. 7, 779–784 (1995).
C. Chen and G. G. Schofield, “Nitric oxide donors enhanced Ca2+ currents and blocked noradrenaline-induced Ca2+ current inhibition in rat sympathetic neurons,”J. Physiol.,482, No. 3, 521–531 (1995).
A. Malgaroll, R. Malinow, H. Schulman, et al., “Persistent signaling and changes in presynaptic function in long-term potentiation,”CIBA Found. Symp.,164, 176–191 (1992).
E. McGlade-McCulloch, H. Yamamoto, S. E. Tan, et al., “Phosphorylation and regulation of glutamate receptors by calcium calmodulin-dependent protein kinase II,”Nature,362, No. 6425, 640–642 (1993).
J. L. Yakel, P. Vissavajihala, V. A. Derkach, et al., “Identification of Ca++/calmodulin-dependent protein kinase II regulatory phosphorylation site in non-N-methyl-D-aspartate glutamate receptors,”Proc. Natl. Acad. Sci. USA,92, No. 5, 1376–1380 (1995).
V. M. Coghlan, B. A. Perrino, M. Howard, et al., “Association of protein kinase A and protein phosphatase 2B with a common anchoring protein,”Science,265, No. 5194, 108–111 (1995).
R. C. Malenka and A. Baskys, “Metabotropic glutamate receptor agonists depress synaptic transmission in area CA1 of the rat hippocampusin vitro,”J. Neurochem,57, No. 1, 560 (1991).
O. V. Garashchuk, Yu. N. Koval'chuk, and O. A. Kryshtal', “Effects of a trans-ACPD-specific glutamate agonist, interacting with metabotropic receptors, on synaptic transmission in the rat hippocampus,”Neirofiziologiya,24 No. 2, 211–214 (1992).
G. L. Collingridge, C. E. Herron, and R. A. J. Lester, “Frequence-dependent N-methyl-D-aspartate receptor-mediated synaptic transmission in rat hippocampus,”J. Physiol.,399, 301–312 (1988).
Z. I. Bashir, Z. A. Bortolotto, C. H. Davies, et al., “Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors,”Nature,363, No. 6427, 347–350 (1993).
L. Aniksztein, P. Bregestovski, and Y. Ben-Ari, “Selective activation of quisqualate metabotropic receptor potentiates NMDA but not AMPA responses,”Eur. J. Pharmacol.,205, No. 3, 327–328 (1991).
T. Behnisch and K. G. Reymann, “Co-activation of metabotropic glutamate and N-methyl-D-aspartate receptors is involved in mechanisms of long-term potentiation maintenance in rat hippocampalCA1 neurons,”Neuroscience,54, No. 1, 37–47 (1993).
S. Alford, B. G. Frenguelli, and G. L. Collingridge, “Ca2+ release from intracellular stores magnifies the Ca2+ signal which permeates dendritic NMDA channels following synaptic activation ofCA1 hippocampal neuronesin vitro.”J. Physiol.,452, 178 (1992).
G. L. Parsadanyan, “Calcineurin of the brain and a group of related type-2B phosphoprotein phosphatases,”Neirokhimiya,9, No. 4, 502–518 (1990).
R. M. Mulkey, S. Endo, S. Shenolikar, et al., “Involvement of calcineurin/inhibitor 1 phosphatase cascade in hippocampal long-term depression,”Nature,369, No. 6480, 486–488 (1993).
H. Kasai and O. H. Petersen, “Spatial dynamics of second messengers: IP3 and cAMP as long-range and associative messengers,”Trends Neurosci.,17, No. 3, 95–101 (1994).
R. Anwyl, “Protein kinase C and long-term potentiation in the hippocampus,”Trends Pharmacol. Sci.,10, No. 6, 236–239 (1989).
G. L. Colligridge, C. H. Davies, and K. G. Raymann, “Long-term potentiation in the rat hippocampal slicesin vitro is associated with a delayed increase in sensitivity ofCA1 neurones to AMPA,”J. Physiol.,414, 23P (1989).
Wang Lu-Yang, E. M. Dudek, M. D. Browning, et al., “Modulation of AMPA/kainate receptors in cultured murine hippocampal neurones by protein kinase C,”J. Physiol.,475, 431–437 (1994).
L. O. Trussel and G. D. Fischbach, “Glutamate receptor desensitization and its role in synaptic transmission,”Neuron,3, No. 2, 209–218 (1989).
Author information
Authors and Affiliations
Additional information
Neirofiziologiya/Neurophysiology, Vol. 28, No. 4/5, pp. 163–172, July–October, 1996.
Rights and permissions
About this article
Cite this article
Abramets, I.I., Samoilovich, I.M. & Kharin, N.A. Postsynaptic mechanisms of induction of NMDA-dependent long-term post-tetanic potentiation of synaptic transmission. Neurophysiology 28, 127–134 (1996). https://doi.org/10.1007/BF02262773
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02262773