Summary
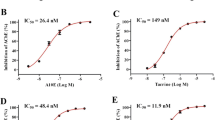
9-Amino-1,2,3,4-tetrahydroaminoacridine (THA) in combination with lecithin has been reported to improve the memory of Alzheimer's disease patients. We have examined some properties of THA in vitro and in vivo so as to define some of the mechanism(s) by which THA might produce its therapeutic effects. In vitro, THA was more potent at inhibiting human plasma cholinesterase (IC50=0.03 μM) than human erythrocyte acetylcholinesterase (IC50=0.3 μM) and rat brain acetylcholinesterase (IC50=0.32 μM). Radioligand binding studies indicated that THA binds reversibly and competitively to primary M 1 and M 2 human cortical muscarinic receptors with similar affinities. Moreover, THA showed similar affinity for temporal cortices muscarinic receptors from Alzheimer and non-Alzheimer (control) brains. In vivo, subcutaneous administration of THA (1–8 mg/kg body weight) to adult rats (6 months old) produced a dose dependent decrease in general activity compared to salinetreated rats. However, at a concentration of 0.5 mg/kg body weight, the general activity of the rats was increased compared to saline-treated rats. The cognitive function of the THA-treated adult rats (subcutaneously 2 mg/kg body weight) was not significantly improved compared to saline-treated rats. It is concluded that the mechanisms of action of THA on the cholinergic system involve reversible inhibition of cholinesterases and reversible and competitive interaction with muscarinic acetylcholine receptors. These effects might be of therapeutic value in the treatment of Alzheimer's disease.
Similar content being viewed by others
References
Adem A, Synnergren B, Botros B, Öhman B, Winblad B, Nordberg A (1987)3H-acetylcholine nicotinic recognition sites in human brain: characterization of agonist binding. Neurosci Lett 83: 298–302
Adem A, Nordberg A, Winblad B (1988) Interference of THA with the binding of muscarinic cholinergic agonists and antagonists to human cortical tissues. In: Proceedings of the international symposium on Alzheimer's disease, June 12–15, Kuopio, Finland
Adem A, Mohammed A, Nordberg A, Winblad B (1990) Tetrahydroaminoacridine and some of its analogues: effects on the cholinergic system. In: Nagatsu T, Fisher A, Yoshida M (eds) Alzheimer's and Parkinson's diseases. II. Basic and therapeutic strategies. Plenum, New York (in press)
Ashkenazi A, Peralta EG, Winslow JW, Ramachandran J, Capon DJ (1988) Functional role of muscarinic acetylcholine receptor subtype diversity. Cold Spring Harb. Symp. Quant. Biol. LIII, pp 263–273
Augustinsson K-B, Eriksson H, Faijersson Y (1978) A new approach to determining cholinesterase activities in samples of whole blood. Clin Chim Acta 89: 239–252
Baeur RH (1982) Age-dependent effects of scopolamine on avoidance, locomotor activity and rearing. Behav Brain Res 5: 261–279
Boyd WD, Graham White J, Blackwood B, Glen I, McQueen J (1977) Clinical effects of choline in Alzheimer senile dementia. Lancet ii: 711
Bryson R, Biner PM, McNair E, Bergondy M, Abrams OR (1981) Effects of nicotine on two types of motor activity in rats. Psychopharmacology 73: 168–170
Campbell BA, Lytle LD, Fibiger HC (1969) Ontogeny of adrenergic arousal and cholinergic inhibitory mechanisms in the rat. Science 166: 635–637
Christie JE, Blackburn IM, Glen AIM, Zeisel S, Shering A, Yates CM (1969) Effect of choline and leicithin on CSF choline levels and on cognitive function in patients with presenile dementia of the Alzheimer's type, In: Barbeau A, Growden JH, Wurtman RJ (eds) Nutrition and the brain, vol 5 Raven Press, New York, pp 389–390
Christie JE, Shering A, Ferguson J, Glenn AIM (1987) Physostigmine and arecoline: effects of intravenous infusions in Alzheimer presenile dementia. Br J Psychiatry 138: 46–50
Davis KI, Mohs RC (1979) Enhancement of memory by physostigmine. N Engl J Med 301: 946
Drukarch B, Kits KS, van der Meer EG, Lodder JC, Stoof JC (1987) 9-Amino-1,2,3,4-tetrahydroacridine (THA), an alleged drug for the treatment of Alzheimer's disease, inhibits acetylcholinesterase activity and slow outward K+ current. Eur J Pharmacol 141: 153–157
Elinder F, Mohammed AK, Winblad B, Århem P (1989) Effects of THA on ionic currents in myelinated axons of xenopus laevis. Eur J Pharmacol 164: 599–602
Etienne P, Gauthier S, Dastoor D, Collier B, Ratner J (1969) Alzheimer's disease: clinical effects of licithin treatment. In: Barbeau A, Growden JH, Wurtman RJ (eds) Nutrition and the brain, vol 5. Raven Press, New York, pp 389–390
Etienne P, Gauthier S, Johnson G, Collier B, Mendis T, Dastoor D, Cole M, Muller HF (1978) Clinical effects of choline in Alzheimer's disease. Lancet i: 508–509
Fibiger HC, Lynch GS, Campbell BA (1970) Cholinergic modulation of adrenergic arousal in the developing rat. J Comp Physiol Psychol 72: 384–389
Fibiger HC, Lynch GS, Cooper HP (1971) A biphasic action of central cholinergic stimulation on behavioural arousal in the rat. Psychopharmacologia 20: 366–382
Flood JF, Cherkin A (1988) Effect of acute arecoline tacrine and arecoline+tacrine post-training administration on retention in old mice. Neurobiol Aging 9: 5–8
Garg M (1969) The effects of some central nervous system stimulant and depressant drugs on rearing activity in rats. Psychopharmacologia 14: 150–156
Ho AKS, Freeman SE (1965) Anticholinesterase activity of tetrahydroaminoacridine and succinyl choline hydrolysis. Nature 205: 1118–1119
Kopelman MD (1986) The cholinergic neurotransmitter system in human memory and dementia: a review. Q J Exp Psychol [A] 38: 535–573
Mazurski EJ, Beninger RJ (1988) Scopolamine produces environment specific conditioned activity that is not blocked by primozide in rats. Psychopharmacology 96: 375–380
Mohammed AK, Jonsson G, Sundström E, Minor BG, Söderberg U, Archer T (1986) Selective attention and place navigation in rats treated prenatally with methylazoxymethanol. Dev Brain Res 30: 145–155
Mohammed AK, Magnusson O, Maehlen J, Fonnum F, Norrby E, Schultzberg M, Kristensson K (1990) Behavioural deficits and serotonin depletion in adult rats after transient infant nasal viral infection. Neuroscience (in press)
Mohammed AK, Wahlström G, Archer T, Nordberg A (1990a) Learning deficits in aged rats pretreated chronically with barbital and tested late in abstinence: alleviation by tetrahydroaminoacridine. (Submitted)
Mohs RC, Davis BM, Greenwald BS, Mathe AA, Johns CA, Horvath TB, Davis KL (1985) Clinical studies on the cholinergic deficit in Alzheimer's disease. II. Psychopharmacological studies. J Am Geriatr Soc 33: 749–757
Nabeshima T, Yoshida S, Kameyana T (1988) Effects of the novel compound NIK-247 on impairment of passive avoidance response in mice. Eur J Pharmacol 154: 263–269
Nilsson L, Adem A, Hardy J, Winblad B, Nordberg A (1987) Do tetrahydroamino acridine (THA) and physostigmine restore acetylcholine release in Alzheimer brains via nicotinic receptors? J Neural Transm 70: 357–368
Nordberg A, Nilsson L, Adem A, Hardy J, Winblad B (1988) Effect of THA on acetylcholine release and cholinergic receptors in Alzheimer brains. In: Giacobini E, Becker R (eds) Current research in Alzheimer therapy. Taylor and Francis, New York, pp 247–257
Nordberg A, Nilsson-Håkansson, Adem A, Lai Z, Winblad B (1990) Multiple actions of THA on cholinergic neurotransmission in Alzheimer brains. In: Iqbal K, Wisniewski H, Winblad B (eds) Proceedings of the First International Conference on Alzheimer's disease and related disorders, Las Vegas, September 6–10, 1988, pp 1169–1178
Nordström Ö, Westlind A, Undén A, Meyerson B, Sachs C, Bartfai T (1982) Pre and post synaptic muscarinic receptors in surgical samples from human cerebral cortex. Brain Res 234: 287–297
Osterrieder W (1987) 9-Amino-1,2,3,4-tetrahydroacridine (THA) is a potent blocker of cardiac potassium channels. Br J Pharmacol 92: 521–525
Pearce BD, Potter L (1988) Effects of tetrahydroaminoacridine on M 1 and M 2 muscarine receptors. Neurosci Lett 88: 281–285
Perry EK, Smith CJ, Court JA, Bonham JR, Rodway M, Atack (1988) Interaction of 9-amino-1,2,3,4-tetrahydroaminoacridine (THA) with human cortical nicotinic and muscarinic receptor binding in vitro. Neurosci Lett 91: 211–216
Peters BH, Levin HS (1979) Effects of physostigmine and lecithin on memory in Alzheimer's disease. Ann Neurol 6: 219–221
Potter LT, Ferrendelli CA, Hanchett HE, Hollifield MA, Lorenzi MV (1989) Tetrahydroaminoacridine and other allosteric antagonists of hippocampal M 1 muscarine receptors. Mol Pharmacol 35: 652–660
Reiner PB, McGeer EG (1988) THA increases action potential duration of central histamine neurons in vitro. Eur J Pharmacol 155: 265–270
Rogawski MA (1987) Tetrahydroaminoacridine blocks voltage-dependent ion channels in hippocampal neurons. Eur J Pharmacol 142: 169–173
Sanberg PR, Henault MA, Hagenmeyer-Houser SH, Russel KH (1987) The topography of amphetamine and scopolamine-induced hyperactivity: toward an activity print. Behav Neurosci 101: 131–133
Schauf CL, Sattin A (1987) Tetrahydroaminoacridine blocks potassium channels and inhibits sodium inactivation in myxicola. J Pharmacol Exp Ther 243: 609–613
Schlatter J, Bättig K (1979) Differential effects of nicotine and amphetamine on locomotor activity and maze exploration in two rat lines. Psychopharmacology 64: 155–161
Sherman KA, Messamore E (1988) Blood cholinesterase inhibition as a guide to the efficacy of putative therapies for Alzheimer's dementia: comparison of tacrine and physostigmine. In: Giacobini E, Becker RE (eds) Current research in Alzheimer therapy. Taylor and Francis, New York, pp 73–86
Sitram N, Weingartner, Gillin JC (1978) Serial learning: enhancement with arecoline and choline and impairment with scopolamine. Science 201: 274–276
Summers WK, Kaufman MR, Altman F, Fisher JM (1980) THA-a review of the literature and its use in treatment of five overdose patients. Clin Toxicol 16: 269–281
Summers WK, Majorski LV, Marsh GM, Tachiki K, Kling A (1986) Oral tetrahydroaminoacridine in long term treatment of senile dementia, Alzheimer type. N Engl J Med 315: 1241–1245
Winer BJ (1971) Statistical principles in experimental designs. McGraw-Hill, New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Adem, A., Mohammed, A.K. & Winblad, B. Multiple effects of tetrahydroaminoacridine on the cholinergic system: Biochemical and behavioural aspects. J Neural Transm Gen Sect 2, 113–128 (1990). https://doi.org/10.1007/BF02260899
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02260899