Summary
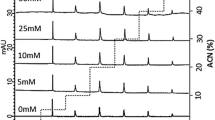
Reversed-phase liquid-liquid chromatographic systems consisting of an aqueous mobile phase and an organic liquid stationary phase of the proton acceptor tri-n-octylphosphine oxide (TOPO) inn-decane, coated on LiChrosorb RP-8, have been studied. The solutes were hydrophilic aromatic carboxylic acids and phenol. The retention of the carboxylic acids shows a minimum at 10 mM of TOPO, whereas increasingly tailing peaks have been obtained with decreasing concentrations of TOPO. This behaviour is due to a concurrent complex formation by hydrogen bonding with TOPO in the liquid stationary phase and adsorption at the interface between the support and the liquid stationary phase. The adsorption of TOPO, ketones and aromatic acids from hexane on Li-Chrosorb RP-8 has been studied, and seems to be due to residual silanol groups. The adsorption isotherm of TOPO has been determined and can be described by a two-site Langmuir adsorption model. Non polar solutes are not adsorbed. The influence of TOPO on the retention and the peak symmetry of carboxylic acids in the liquid-liquid chromatographic system appears to be due to a competition between TOPO and the acids for the same adsorption sites. No competition was found for phenol.
Similar content being viewed by others
Abbreviations
- a:
-
adsorbing phase
- m:
-
mobile phase
- s:
-
liquid stationary phase
- CHX :
-
total concentration of HX (M or mol/g)
- [HX]:
-
concentration of the species HX (M or mol/g)
- DHX(a) :
-
distribution ratio of HX between the adsorbing phase and the mobile organic phase (L/g), defined by eq. (11)
- D′HX(s) :
-
distribution ratio of HX between the adsorbing phase and the mobile aqueous phase (L/g), defined by eq. (14)
- DHX(s) :
-
distribution ratio of HX between the liquid stationary phase and the mobile phase, defined by eq. (4)
- KD(HX) :
-
distribution constant of HX, defined by eq. (5)
- KHX-TOPO n :
-
equilibrium constant for the formation of the complex between HX andn TOPO molecules (M−n), defined by eq. (6)
- KHX(A) :
-
equilibrium constant for the adsorption of HX on adsorbing sites A (M−1), defined by eq. (8)
- KO(A) :
-
monolayer capacity of sites A on the adsorbent (mol/g)
- Vm :
-
volume of the mobile phase (L)
- Vs :
-
volume of the liquid stationary phase (L)
- VR(TOPO) :
-
retention volume of TOPO(L)
- W:
-
weight of adsorbent (g)
- εm:
-
porosity of the column
References
H. W. Stuurman, K.-G. Wahlund, G. Schill, J. Chromatogr.204, 43 (1981).
H. W. Stuurman, K.-G. Wahlund, J. Chromatogr.218, 455 (1981).
O. Gyllenhaal, H. W. Stuurman, J. Chromatogr.248, 109 (1982).
J. C. Giddings, Dynamics of Chromatography, Part I, Marcel Dekker, New York, 1965.
Ya. I. Korenman, T. P. Koroleva, Zh. Prikl. Khim. (Leningrad)48, 1413 (1975); Chem. Abstr.83, 104119a (1975).
A. Tilly-Melin, Y. Askemark, K.-G. Wahlund, G. Schill, Anal. Chem.51, 976 (1979).
L. R. Snyder, Principles of Adsorption Chromatography, Marcel Dekker, New York, 1968.
G. Aksnes, T. Gramstad, Acta Chem. Scand.14, 1485 (1960).
M. Schröder-Nielsen, Acta Pharm. Suec.13, 439 (1976).
R. P. W. Scott, P. Kucera, J. Chromatogr.149, 93 (1978).
P. Roumeliotis, K. K. Unger, J. Chromatogr.149, 211 (1978).
N. Tanaka, H. Goodell, B. L. Karger, J. Chromatogr.158, 233 (1978).
K. Karch, I. Sebastian, I. Halász, J. Chromatogr.122, 3 (1976).
K. K. Unger, N. Becker, P. Roumeliotis, J. Chromatogr.125, 115 (1976).
G. E. Berendsen, K. A. Pikaart, L. de Galan, J. Liq. Chromatogr.3, 1437 (1980).
J. F. K. Huber, R. G. Gerritse, J. Chromatogr.58, 137 (1971).
H. W. Stuurman, E. Arvidsson, in preparation.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stuurman, H.W., Wahlund, K.G. Concurrent adsorption and complex formation in reversed-phase liquid-liquid chromatography with tri-n-octylphosphine oxide. Chromatographia 16, 147–151 (1982). https://doi.org/10.1007/BF02258885
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02258885