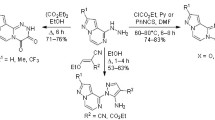
Abstract
The thermodynamic stability of the antiaromatic 4,8-dihydrodifurazano[3,4-b,e]pyrazine (I) was studied by a quantum-chemical method. The molecular structure was investigated by x-ray crystallographic analysis, and the aromaticity index of the compound was calculated. It was shown that the oxidation or nitration of compound (I) leads to a stable aromatic radical.
Similar content being viewed by others
References
L. Willer and D. W. Moore, J. Org. Chem.,50, 5123 (1985).
R. L. Willer, US Patent No. 4503229; Chem. Abs.,103, 54099c (1986).
R. L. Willer, US Patent No. 4539405; Ref. Zh. Khim.,12, N224P (1986).
A. V. Eremeev, I. B. Starchenko, and V. G. Andrianov, “4,8-Dihydrodifurazano[3,4-b, e]pyrazine — a new heterocyclic system,” Inventor's Certificate No. 320130 (1986).
A. V. Eremeev, I. B. Starchenko, and V. G. Andrianov, “Method for the Production of 4,8-Dihydrodifurazano[3,4-b,e]pyrazine,” Inventor's Certificate No. 288455 (1987).
I. B. Starchenko, Thesis for Candidate, of Chemical Sciences [in Russian], Riga (1989).
I. B. Starchenko and V. T. Andrianov, Khim. Geterotsikl. Soedin., No. 5, 717 (1996).
W. Kaim, J. Mol. Struct. (THEOCHEM),109, 277 (1984).
M. J. S. Dewar and W. Thiel, J. Am. Chem. Soc.,99, 4907 (1977).
A. S. Batsanov and Yu. T. Struchkov, Zh. Strukt. Khim.,26, 65 (1985).
L. Tauscher, S. Ghisla, and P. Hemmerich, Helv. Chim. Acta,56, 630 (1973).
C. W. Bird, Tetrahedron,41, 1409.
W. Kaim, J. Chem. Soc. Perkin Trans. II, 1633 (1985).
M. D. Boldyrev and B. V. Gidaspov, Procedural Hints in Laboratory Practise [in Russian], Leningr. Tekhnol. Inst. Lensovet (1978), p. 16.
J. W. Fischer, R. A. Nissan, and S. K. Lowe-Ma, J. Heterocycl. Chem.,28, 1677 (1991).
Additional information
Latvian Institute of Organic Synthesis, Riga LV-1006. Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 2, pp. 250–264, February 1997.
Rights and permissions
About this article
Cite this article
Starchenkov, I.B., Andrianov, V.G. & Mishnev, A.F. Chemistry of furazano[3,4-b]pyrazine. 1. Synthesis and thermodynamic appraisal of 4,8-dihydrodifurazano[3,4-b,e]pyrazine and its derivatives. Chem Heterocycl Compd 33, 216–228 (1997). https://doi.org/10.1007/BF02256764
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02256764