Abstract
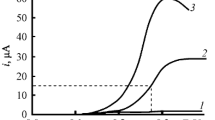
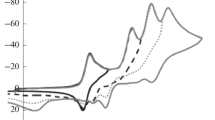
The characteristics (potentials, limiting currents, reversibility) of the electrochemical reduction of 3,5-dinitro-1,2-dihydropyridines were determined by cyclic voltammetry and polarography. It was established by ESR that the free radicals formed in these processes have a radical-dianion structure in the case of both the N-unsubstituted and the N-substituted dihydropyridines. The hyperfine coupling constants of the interaction between the unpaired electron and the nuclei of the atoms at various positions of the heterocycle were determined.
Similar content being viewed by others
References
B. Kastening, Progress of Polarography,3, 195 (1972).
Ya. Stradyn', R. Gavars, and L. Baumane, Élektrokhimiya,31, No. 10, 1100 (1995).
J. Stradins, J. Ogle, V. Kadysh, L. Baumane, R. Gavars, and G. Duburs, J. Electroanalyt. Chem.,226, 103 (1987).
Ya. V. Ogle, L. Kh. Baumane, Ya. P. Stradyn', G. Ya. Dubur, B. P. Kadysh, R. A. Gavar, and V. K. Lusis, Khim Geterotsikl. Soedin., No. 8, 1099 (1985).
L. Kh. Baumane, Ya. P. Stradyn', R. A. Gavar, A. P. Gaukhman, and G. Ya. Dubur, Khim. Geterotsikl. Soedin., No. 11, 1494 (1988).
L. Kh. Baumane, Ya. P. Stradyn', R. A. Gavar, B. S. Chekavichus, and G. Ya. Dubur, Khim. Geterotsikl. Soedin., No. 4, 481 (1991).
L. Baumane, J. Stradins, R. Gavars, and G. Duburs, Electrochim. Acta,37, No. 14, 2599 (1992).
Ya. Stradyn', L. Baumane, R. Gavars, B. Chekavichus, and G. Duburs, Khim. Geterotsikl. Soedin., No. 11, 1498 (1992).
Ya. Stradyn', L. Baumane, R. Gavars, B. Vigante, and G. Duburs, Khim. Geterotsikl. Soedin., No. 3, 355 (1995).
Ya. Stradyn', P. Gavars, L. Baumane, B. Vigante, and G. Duburs, Khim. Geterotsikl. Soedin., No. 9, 1222 (1996).
Ya. Stradyn', L. Baumane, R. Gavars, B. Vigante, and G. Duburs, Khim. Geterotsikl. Soedin., No. 7, 936 (1996).
Ya. Stradyn', R. Gavars, L. Baumane, B. Vigante, and G. Duburs, Khim. Geterotsikl. Soedin., No. 8, 1079 (1993).
T. Fujinaga, Y. Degushi, and K. Uemoto, Bull. Chem. Soc. Jpn.,37, No. 6, 822 (1964).
M. Barzaghi, A. Gamba, G. Morosi, and M. Simoneta, J. Phys. Chem.,78, No. 1, 49 (1974).
Yu. M. Kargin, V. V. Kondranina, and N. I. Semakhina, Izv. Akad. Nauk. Ser. Khim., No. 2, 278 (1971).
B. Vigante, Ya. Ozols, and G. Dubur, Khim. Geterotsikl. Soedin., No. 1, 64 (1993).
Additional information
Latvian Institute of Organic Synthesis, Riga LV-1006. Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 2, pp. 212–218, February, 1997.
Rights and permissions
About this article
Cite this article
Stradyn', Y., Gavar, R., Baumane, L. et al. Free radicals in the electrochemical reduction of derivatives of 3,5-dinitro-1,2-dihydropyridine. Chem Heterocycl Compd 33, 184–189 (1997). https://doi.org/10.1007/BF02256760
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02256760