Abstract
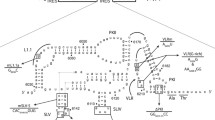
RPE. 40 mutant cells differ from wild-type Chinese hamster ovary (CHO-K1) cells in their increased resistance toPseudomonas exotoxin A and their inability to process the insulin proreceptor and certain viral envelope proproteins. Northern analysis revealed that RPE. 40 cells maintained a substantially lower steady-state level of 4.0 kbfur mRNA than did CHO-K1 cells. Analysis offur cDNAs showed that RPE. 40 cells were diploid at thefur locus, and RPE. 40 cells had a Cys (TGC) to Tyr (TAC) mutation in codon 196 of one allele (allele I). Approximately 25–30% of the CHO-K1 cells were also heterozygous (Tyr/Cys) at codon 196, and pre-mRNAs transcribed from the second allele (allele II) in RPE. 40 cells were defectively spliced. All other pre-mRNAs were correctly spliced. Rapid turnover of defectively spliced transcripts may account for the reduced steady-state level offur mRNA observed in RPE. 40 cells. Our results provide a mechanistic basis for the endoprotease-deficient phenotype of RPE. 40 cells.
Similar content being viewed by others
Literature Cited
Steiner, D.F., and Oyer, P.E. (1967).Proc. Natl. Acad. Sci. U.S.A. 57:473–480.
Docherty, K., and Steiner, D.F. (1982).Annu. Rev. Physiol. 44:625–638.
Douglas, J., Civelli, O., Herbert, E. (1984).Annu. Rev. Biochem. 53:665–715.
Harris, R.B. (1989).Arch. Biochem. Biophys. 275:315–333.
Steiner, D.F., Smeekens, S.P., Ohagi, S., and Chan, S.J. (1992).J. Biol. Chem. 267:23435–23438.
Bresnahan, P.A., Hayflick, J.S., Molloy, S.S., and Thomas, G. (1993). InMechanisms of Intracellular Trafficking and Processing of Pro-proteins, (ed.) Loh, Y.P. (CRC Press Inc., Boca Raton, Florida), pp. 225–250.
Sossin, W.S., Fisher, J.M., and Scheller, R.H. (1989).Neuron 2:1407–1417.
Barr, P.J. (1991).Cell 66:1–3.
McCune, J.M., Rabin, L.B., Feinberg, M.B., Lieberman, M., Kosek, J.C., Reyes, G.R., and Weissman, I.L. (1988).Cell 53:55–67.
Stein, B.S., and Engleman, E.G. (1990).J. Biol. Chem. 265:2640–2649.
Watson, D.G., Moehring, J.M., and Moehring, T.J. (1991).J. Virol. 65:2332–2339.
Inocencio, N.M., Moehring, J.M., and Moehring, T.J. (1993).J. Virol. 67:593–595.
Hallenberger, S., Bosch, V., Angliker, H., Shaw, E., Klenk, H.-D., and Garten, W. (1992).Nature 360:358–361.
Stieneke-Grober, A., Vey, M., Angliker, H., Shaw, E., Thomas, G., Roberts, C., Klenk, H.-D., and Garten, W. (1992).EMBO J. 11:2407–2414.
Hosaka, M., Nagahama, M., Kim, W.-S., Watanabe, T., Hatsuzawa, K., Ikemizu, J., Murakami, K., and Nakayama, K. (1991).J. Biol. Chem. 266:12127–12130.
Molloy, S.S., Bresnahan, P.A., Leppla, S.H., Klimpel, K.R., and Thomas, G. (1992).J. Biol. Chem. 267:16396–16402.
Julius, D., Brake, A., Blair, L., Kunisawa, R., and Thorner, J. (1984).Cell 37:1075–1089.
Fuller, R.S., Brake, A.J., and Thorner, J. (1989).Proc. Natl. Acad. Sci. U.S.A. 86:1434–1438.
Fuller, R.S., Brake, A.J., and Thorner, J. (1989).Science 246:482–486.
Roebroek, A.J.M., Schalken, J.A., Leunissen, J.A.M., Onnekink, C., Bloemers, H.P.J., and Van de Ven, W.J.M. (1986).EMBO J. 5:2197–2202.
Misumi, Y., Sohda, M., and Ikehara, Y. (1990).Nucleic Acids Res. 18:6719.
Hatsuzawa, K., Hosaka, M., Nakagawa, T., Nagase, M., Shoda, A., Murakami, K., and Nakayama, K. (1990).J. Biol. Chem. 265:22075–22078.
Korner, J., Chun, J., O'Bryan, L., and Axel, R. (1991).Proc. Natl. Acad. Sci. U.S.A. 88:11393–11397.
Roebroek, A.J.M., Creemers, J.W.M., Pauli, I.G.L., Bogaert, T., and Van de Ven, W.J.M. (1993).EMBO J. 12:1853–1870.
Roebroek, A.J.M., Creemers, J.W.M., Pauli, I.G.L., Kurzik-Dumke, U., Rentrop, M., Gateff, E.A.F., Leunissen, J.A.M., and Van de Ven, W.J.M. (1992).J. Biol. Chem. 267:17208–17215.
Bresnahan, P.A., Leduc, R., Thomas, L., Thorner, J., Gibson, H.L., Brake, A.J., Barr, P.J., and Thomas, G. (1990).J. Cell Biol. 111:2851–2859.
Rehemtulla, A., Dorner, A.J., and Kaufman, R.J. (1992).Proc. Natl. Acad. Sci. U.S.A. 89:8235–8239.
Molloy, S.S., Thomas, L., Van Slyke, J.K., Stenberg, P.E., and Thomas, G. (1994).EMBO J. 13:18–33.
Van de Ven, W.J.M., Voorberg, J., Fontijn, R., Pannekoek, H., Van den Ouweland, A.M.W., Van Duijnhoven, H.L.P., Roebroek, A.J.M., and Siezen, R.J. (1990).Mol. Biol. Rep. 14:265–275.
Leduc, R., Molloy, S.S., Thorne, B.A., and Thomas, G. (1992).J. Biol. Chem. 267:14304–14308.
Kiefer, M.C., Tucker, J.E., Joh, R., Landsberg, K.E., Saltman, D., and Barr, P.J. (1991).DNA Cell Biol. 10:757–769.
Seidah, N.G., Gaspar, L., Mion, P., Marcinkiewicz, M., Mbikay, M., and Chretien, M. (1990).DNA Cell Biol. 9:415–424.
Smeekens, S.P., and Steiner, D.F. (1990).J. Biol. Chem. 265:2997–3000.
Hakes, D.J., Birch, N.P., Mezey, A., and Dixon, J.E. (1991).Endocrinology 129:3053–3063.
Nakayama, K., Kim, W.-S., Torii, S., Hosaka, M., Nakagawa, T., Ikemizu, J., Baba, T., and Murakami, K. (1992).J. Biol. Chem. 267:5897–5900.
Nakagawa, T., Hosaka, M., Torii, S., Watanabe, T., Murakami, K., and Nakayama, K. (1993).J. Biochem. 113:132–135.
Creemers, J.W.M., Siezen, R.J., Roebroek, A.J.M., Ayoubi, T.A.Y., Huylebroeck, D., and Van de Ven, W.J.M. (1993).J. Biol. Chem. 268:21826–21834.
Robertson, B.J., Moehring, J.M., and Moehring, T.J. (1993).J. Biol. Chem. 268:24274–24277.
Wise, R.J., Barr, P.J., Wong, P.A., Kiefer, M.C., Brake, A.J., and Kaufman, R.J. (1990).Proc. Natl. Acad. Sci. U.S.A. 87:9378–9382.
Misumi, Y., Oda, K., Fujiwara, T., Takami, N., Tashiro, K., and Ikehara, Y. (1991).J. Biol. Chem. 266:16954–16959.
Wasley, L.C., Rehemtulla, A., Bristol, J.A., and Kaufman, R.J. (1993).J. Biol. Chem. 268:8458–8465.
Klimpel, K.R., Molloy, S.S., Thomas, G., and Leppla, S.H. (1992).Proc. Natl. Acad. Sci. U.S.A. 89:10277–10281.
Moehring, J.M., Inocencio, N.M., Robertson, B.J., and Moehring, T.J. (1993).J. Biol. Chem. 268:2590–2594.
Jiang, Y.-P., Wang, H., D'Eustachio, P., Musachio, J.M., Schlessinger, J., and Sap, J. (1993).Mol. Cell. Biol. 13:2942–2951.
Kayyem, J.F., Roman, J.M., de la Rosa, E.J., Schwarz, U., and Dreyer, W.J. (1992).J. Cell Biol. 118:1259–1270.
Moehring, J.M., and Moehring, T.J. (1983).Infect. Immun. 41:998–1009.
Mondino, A., Giordano, S., and Comoglio, P.M. (1991).Mol. Cell. Biol. 11:6084–6092.
Komada, M., Hatsuzawa, K., Shibamoto, S., Ito, F., Nakayama, K., and Kitamura, N. (1993).FEBS Lett. 195:1019–1026.
Takahashi, S., Kasai, K., Hatsuzawa, K., Kitamura, N., Misumi, Y., Ikehara, Y., Murakami, K., and Nakayama, K. (1993).Biochem. Biophys. Res. Commun. 195:1019–1026.
Inocencio, N.M., Moehring, J.M., and Moehring, T.J. (1994).J. Biol. Chem. 269:31831–31835.
Moehring, J.M., and Moehring, T.J. (1979).Somat. Cell Genet. 5:453–468.
Peppel, K., and Baglioni, C. (1990).Biotechniques 9:711–713.
Maniatis, T., Fritsch, E.F., and Sambrook, J. (1982).Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor Laboratory, Cold Spring Harbor, New York).
Virca, G.D., Northemann, W., Shiels, B.R., Widera, G., and Broome, S. (1990).Biotechniques 8:370–371.
Krawczak, M., Reiss, J., and Cooper, D.N. (1992).Hum. Genet. 90:41–54.
Steingrimsdottir, H., Rowley, G., Dorado, G., Cole, J., and Lehmann, A.R. (1992).Nucleic Acids Res. 20:1201–1208.
Zhang, L.-H., Vrieling, H., van Zeeland, A.A., and Jenssen, D. (1992).J. Mol. Biol. 223:627–635.
Carothers, A.M., Urlaub, G., Grunberger, D., and Chasin, L.A. (1993).Mol. Cell. Biol. 13:5085–5098.
Fisher, C.W., Fisher, C.R., Chuang, J.L., Lau, K.S., Chuang, D.T., and Cox, R.P. (1993).Am. J. Hum. Genet. 52:414–424.
Huang, C.-H., Reid, M.E., and Blumenfeld, O.O. (1993).J. Biol. Chem. 268:4945–4952.
Schneider, S., Wildhardt, G., Ludwig, R., and Royer-Pokora, B. (1993).Hum. Genet. 91:599–604.
Kuivaniemi, H., Kontusaari, S., Tromp, G., Zhao, M., Sabol, C., and Prockop, D.J. (1990).J. Biol. Chem. 265:12067–12074.
Overbergh, L., Torrekens, S., Van Leuven, F., and Van den Berghe, H. (1991).J. Biol. Chem. 266:16903–16910.
Patterson, D., Berger, R., Bleskan, J., Vannais, D., and Davidson, J. (1992).Somat. Cell Mol. Genet. 18:65–75.
Epstein, D.J., Vogan, K.J., Trasler, D.G., and Gros, P. (1993).Proc. Natl. Acad. Sci. U.S.A. 90:532–536.
Baserga, S.J., and Benz, E.J., Jr. (1988).Proc. Natl. Acad. Sci. U.S.A. 85:2056–2060.
Daar, I.O., and Maquat, L.E. (1988).Mol. Cell. Biol. 8:802–813.
Brawerman, G. (1989).Cell 57:9–10.
Hatsuzawa, K., Murakami, K., and Nakayama, K. (1992).J. Biochem. 111:296–301.
Author information
Authors and Affiliations
Additional information
This work was supported by National Institutes of Health Grant AI 09100 and the Lucille P. Markey Charitable Trust.
Rights and permissions
About this article
Cite this article
Spence, M.J., Sucic, J.F., Foley, B.T. et al. Analysis of mutations in alleles of thefur gene from an endoprotease-deficient chinese hamster ovary cell strain. Somat Cell Mol Genet 21, 1–18 (1995). https://doi.org/10.1007/BF02255818
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02255818