Abstract
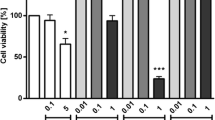
Some components of natural foods may enhance or inhibit prostaglandin formation and potentially affect the inflammation condition. A macrophage cell line, RAW264.7, was employed to examine the effects of foods traditionally regarded as ‘heating’ or ‘cooling’ on the production of PGE2, a well-known proinflammatory mediator. Foods traditionally regarded as ‘heating’ (litchi, longan, and dried longan) or ‘cooling’ (chrysanthemum flower, bitter gourd, and lotus seed plumule) were extracted sequentially with water and ethyl acetate. The water extracts (WE) and ethyl acetate extracts (EAE) were applied to RAW264.7 macrophages in the presence or absence of LPS (lipopolysaccharide). In the absence of LPS, the WEs from the ‘heating foods’, litchi, longan, or dried longan had a dose-dependent enhancing effect on PGE2 production, with respective EC50s of 8.4, 16, and 11 mg/ml. This effect was accompanied by significant induction of COX-2 protein expression, as shown by Western blot analysis. In contrast, LPS-induced PGE2 production was inhibited in a dose-dependent manner by the WEs of the ‘cooling foods’, chrysanthemum flower, bitter gourd, and lotus seed plumule, with respective IC50s of 0.6, 0.13, and 0.08 mg/ml. At the concentrations tested, none of the EAEs had any effect on basal PGE2 production, while LPS-induced PGE2 production was inhibited or increased by the EAE from bitter gourd and longan, respectively. Water-soluble extracts of foods traditionally regarded as ‘heating’ enhanced basal PGE2 production, while those from ‘cooling’ foods significantly inhibited LPS-induced PGE2 production by the macrophage cell line. This subject merits further study to determine whether appropriate food selection may help patients suffering from chronic inflammatory conditions.
Similar content being viewed by others
References
Ammon HP, Safayhi H, Mack T, Sabieraj J. Mechanism of anti- inflammatory actions of curcumine and boswellic acids. J Ethnopharmacol 38:113–119;1993.
Anderson EN Jr. ‘Heating ‘and ‘cooling’ foods in Hong Kong and Taiwan. Soc Sci Inf 19:237–268;1980.
Anderson EN Jr. ‘Heating and cooling’ foods re-examined. Soc Sci Med 23:755–773;1984.
Anderson EN Jr. Why is humoral medicine so popular? Soc Sci Med 25:331–337;1987.
Badawi AF, El-Sohemy A, Stephen LL, Ghoshal AK, Archer MC. The effect of dietary n-3 and n-6 polyunsaturated fatty acids on the expression of cyclooxygenase 1 and 2 and levels of p21ras in rat mammary glands. Carcinogenesis 19:905–910;1998.
Barrios-Rodiles M, Keller K, Belley A, Chadee K. Nonsteroidal anti-inflammatory drugs inhibit cyclooxygenase-2 enzyme activity but not mRNA expression in human macrophages. Biochem Biophys Res Commun 225:896–900;1996.
Briggs GM, Calloway DH. Nutrition and Physical Fitness, ed 11. Philadelphia, Saunders, 1984, chap 19, pp 481–494.
Callejas NA, Castrillo A, Bosca L, Martin-Sanz P. Inhibition of prostaglandin synthesis up-regulates cyclooxygenase-2 induced by lipopolysaccharide and peroxisomal proliferators. J Pharmacol Exp Ther 288:1235–1241;1998.
Chinery R, Beauchamp RD, Shyr Y, Kirkland SC, Coffey, RJ, Morrow JD. Antioxidants reduce cyclooxygenase-2 expression, prostaglandin production, and proliferation in colorectal cancer cells. Cancer Res 58:2323–2327;1998.
D'Acquisto FD, Iuvone T, Rombola L, Sautebin L, Rosa MD, Carnuccio R. Involvement of NF-κB in the regulation of cyclooxygenase-2 protein expression in LPS-stimulated J774 macrophages. FEBS Lett 418:175–178;1997.
Dewitt DL, Meade EA, Smith WL. PGH synthase isozyme selectivity: The potential for safer nonsteroidal anti-inflammatory drugs. Am J Med 95:40S-44S;1993.
Glaser KB, Sung A, Bauer J, Weichman BM. Regulation of eicosanoid biosynthesis in the macrophage. Involvement of protein tyrosine phosphorylation and modulation by selective protein tyrosine kinase inhibitors. Biochem Pharmacol 45:711–721;1993.
Gordon S. The role of the macrophage in immune regulation. Res Immunol 149:685–688;1998.
Gould-Martin K. Hot cold poison and dirt: Chinese folk medical categories. Soc Sci Med 12:39–46;1978.
Herschman HR. Regulation of prostaglandin synthase-1 and prostaglandin synthase-2. Cancer Metastasis Rev 13:241–256;1994.
Hoenig S, Skutelsky E, Leibovici J, Barot R. Changes in distribution of lectin receptors in macrophages activated by Nocardia water-soluble molecules. Cell Mol Biol 39:843–848;1993.
Humes JL, Bonney RJ, Pelus L, Dahlgren ME, Kuehl FLA, Davies P. Macrophages synthesize and release prostaglandins in response to inflammatory stimuli. Nature 269:149–151;1977.
Hwang D, Jang BC, Yu G, Bludreau M. Expression of mitogen-inducible cyclooxygenase induced by lipopolysaccharide. Mediation through both mitogen-activated protein kinase and NF-κB signaling pathways in macrophages. Biochem Pharmacol 54:87–96;1997.
Hwang D, Fischer NH, Jang BC, Tak H, Kim JK, Lee W. Inhibition of the expression of inducible cyclooxygenase and proinflammatory cytokines by sesquiterpine lactones in macrophages correlates with the inhibition of MAP kinases. Biochem Biophys Res Commun 226:810–818;1996.
Johnson JL, Maddipati KR. Paradoxical effects of resveratrol on the two prostaglandin H synthases. Prostaglandins Other Lipid Mediat 56:131–143;1998.
Kimura Y, Minami Y, Tokuda T, Nakajima S, Takagi S, Funatsu G. Primary structures of N-linked oligosaccharides of momordin-a, a ribosome-inactivating protein fromMomordica charantia seeds. Agric Biol Chem 55:2031–2036;1991.
Koo LC. The use of food to treat and prevent disease in Chinese culture. Soc Sci Med 18:757–766;1984.
Krugluger W, Lucas T, Koller M, Boltz-Nitulescu G, Froster O. Soybean agglutinin binds a 160-kDa rat macrophage membrane glycoprotein and enhances cell differentiation and activation. Immunol Lett 52:53–56;1996.
Ku KM, Sansores-Garcia L, Chen XM, Matijevic-Aleksic, N, Du M, Wu KK. Suppression of inducible cyclooxygenase 2 gene transcription by aspirin and sodium salicylate. Proc Natl Acad Sci USA 96:5292–5297;1999.
Kusamran WR, Ratanavila A, Tepsuwan A. Effects of neem flowers, Thai and Chinese bitter gourd fruits and sweet basil leaves on hepatic monooxygenases and glutathione S-transferase activities, and in vitro metabolic activation of chemical carcinogens in rats. Food Chem Toxicol 122:121–126;1998.
Lai CC, Lin BF, Lin KW. Effects of high dietary fat and deteriorated frying oil on prostaglandin E2 production in BALB/c mice. J Chin Agric Chem Soc 35:401–412;1997.
Lands WEM. The biosynthesis and metabolism of prostaglandins. Annu Rev Physiol 41:633–652;1979.
Lee-Huang S, Huang PL, Chen HC, Bourinbaiar A, Huang HI, Kuang HF. Anti-HIV and anti-tumor activities of recombinant MAP30 from bitter melon. Gene 161:151–156;1995.
Liang YC, Huang YT, Tsai SH, Lin-Shiau SY, Chen CF, Lin JK. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophage. Carcinogenesis 20:1945–1952;1999.
Li Y, Matsuda H, Yamahara J, Yoshikawa M. Acceleration of gastrointestinal transit by momordin Ic in mice: Possible involvement of 5-hydroxytryptamine, 5-HT(2) receptors and prostaglandins. Eur J Pharmacol 392:71–77;2000.
Lo CJ, Cryer HG, Fu M, Lo FR. Regulation of macrophage eicosanoid generation is dependent on nuclear factor κB. J Trauma 45:19–24;1998.
Lokesh BR, Kinsella JE. Modulation of prostaglandin synthesis in mouse peritoneal macrophages by enrichment of lipids with either eicosapentaenoic or docosahexaenoic acids in vitro. Immunobiology 175:406–419;1987.
Ludman EK, Newman JM. Yin and yang in the health-related food practices of three Chinese groups. J Nutr Educ 19:3–5;1984.
Ma CC. Chung I Chen Tuan Hseueh (Diagnosis in Chinese Traditional Medicine). Taipei, Kuo Lee Bian I Kuan. 139;1980.
Martinez J, Moreno JJ. Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem Pharmacol 59:865–870;2000.
Matsuda H, Li Y, Murakami T, Matsumura N, Yamahara J, Yoshikawa M. Antidiabetic principles of natural medicines. III. Structure-related inhibitory activity and action mode of oleanolic acid glycosides on hypoglycemic activity. Chem Pharm Bull 46:1399–1403;1998.
Mendez C, Garcia I, Maier R. Antioxidants attenuate endotoxin-induced activation of alveolar macrophages. Surgery 118:412–420;1995.
Miwa M, Kong ZL, Shinohara K, Watanabe M. Macrophage stimulating activity of foods. Agric Biol Chem 54:1863–1866;1990.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assay. J Immunol Methods 65:55–63;1983.
Pang L, Hoult JR. Induction of cyclooxygenase and nitric oxide synthase in endotoxin-activated J774 macrophages is differentially regulated by indomethacin: Enhanced cyclooxygenase-2 protein expression but reduction of inducible nitric oxide synthase. Eur J Pharmacol 317:151–155;1996.
Peterson J, Dwyer J. Flavonoids: Dietary occurrence and biochemical activity. Nutr Res 18:1995–2018;1998.
Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LBA, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J 12:1063–1073;1998.
Pu Z, Lu BY, Liu WY, Jin SW. Characterization of enzymatic mechanism of gamma-momorcharin, a novel ribosome-inactivating protein with lower molecular weight of 11,500 purified from the seeds of bitter gourd (Momordica charantia). Biochem Biophys Res Commun 229:287–294;1996.
Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956;1996.
Riese J, Hoff T, Nordhoff A, Dewitt DL, Resch K, Kaever V. Transient expression of prostaglandin endoperoxide synthase-2 during mouse macrophage activation. J Leukocyte Biol 55:476–482;1994.
Sarkar S, Pranava M, Marita R. Demonstration of the hypoglycemic action ofMomordica charantia in a validated animal model of diabetes. Pharmacol Res 33:1–4;1996.
Shin NH, Ryu SY, Lee H, Min KR, Kim Y. Inhibitory effects of hydroxystilbenes on cyclooxygenase from sheep seminal vesicles. Planta Med 64:283–284;1998.
Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenase)-1 and -2. J Biol Chem 271:33157–33160;1996.
Stock JD, Sansom SC. Regulation of filtration rate by glomerular mesangial cells in health and diabetic renal disease. Am J Kidney Dis 29:971–981;1997.
Subbaramaial K, Quing W, Michaluart P, Telang N, Tanabe T, Inoue H, Jang M, Pezzuto JM, Dannenberg AJ. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem 273:21875–21882;1998.
Sweet MJ, Hume DA. Endotoxin signal transduction in macrophages. J Leukocyte Biol 60:8–26;1996.
Tetsuka T, Baier LD, Morrison AR. Antioxidants inhibit interleukin-1 induced cyclooxygenase and nitric oxide synthase expression in rat mesangial cells. Evidences for post-transcriptional regulation. J Biol Chem 271:11689–11693;1996.
Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature 231:232–235;1971.
Wu MC, Huang, CJ. Inhibition of prostaglandin E2 production of a macrophage cell line by some phytochemicals. Food Sci Agric Chem 3:59–71;2001.
Yamamoto S. Characterization of enzymes in prostanoid synthesis. In: Curtis-Prior PB, ed. Prostaglandins: Biology and Chemistry of Prostaglandins and Related Eicosanoids. Edinburgh, Churchill Livingstone. 37–51;1988.
Yoshinkawa M, Morikawa T, Toguchida I, Harima S, Matsuda H. Medicinal flowers. II. Inhibitors of nitric oxide production and absolute stereostructures of five new germacrane-type sesquiterpenes, kikkanols D, D monoacetate, E, F, and F monoacetate from the flowers ofChrysanthemum indicum L. Chem Pharm Bull 48:651–656;2000.
Zor U, Reiss N. Regulation of plasma membrane phospholipase A2 activity by phosphorylation/dephosphorylation: Is glucocorticoid action mediated by induction of protein phosphatase. In: Bailey JM, ed. Prostaglandins, Leukotrienes, Lipoxins and PAF. New York, Plenum Press. 135–139;1991.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Huang, Cj., Wu, MC. Differential effects of foods traditionally regarded as ‘heating’ and ‘cooling’ on prostaglandin E2 production by a macrophage cell line. J Biomed Sci 9, 596–606 (2002). https://doi.org/10.1007/BF02254987
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02254987