Abstract
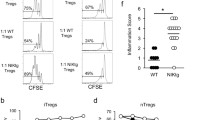
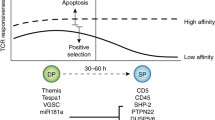
Studies of several gene knockout mice suggest an interesting association of a moderate T cell response with systemic autoimmune diseases. In addition, CD95 ligand (FasL) expression in some strains of these mice is impaired. Because FasL is critically involved in regulating peripheral tolerance, there may be a link between autoimmune diseases and a moderate T cell response that cannot activate the FasL gene. Here, we propose that there are two thresholds of T cell activation. When moderately stimulated, T cells can be activated to the low (1st) threshold, which permits the induction of CD40L, IL-2, IL-4, and other components that help the immune response. The high (2nd) activation threshold can only be achieved by a strong and concurrent stimulation through TCR and IL-2R. Once the high threshold is reached, FasL is produced to induce apoptosis of the activated T and B cells. In the absence of the FasL-mediated downregulation, the activated B cells become efficient antigen-presenting cells for self-antigens and excellent responders for T cell help. Such an exacerbating condition, induced by recurrent and moderate activation, favors the development of autoreactive T cells and autoantibody production. Evidence supporting this hypothesis and some predictions that can be tested are described.
Similar content being viewed by others
References
Andrews, BS, Eisenberg RA, Theofilopoulus AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ. Spontaneous murine lupus-like syndromes. J Exp Med 148:1198–1215;1978.
Ansar AS, Talal N. Immune-endocrine interactions and autoimmune diseases. In: Kammuler ME, Bloksma N, Seinen W. eds. Autoimmunity and Toxicology. Amsterdam, Elsevier, 415–428;1989.
Boehme SA, Lenardo MJ. Propiocidal apoptosis of mature T lymphocytes occurs at S phase of the cell cycle. Eur J Immunol 23:1552–1560;1993.
Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahoubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green DR. Cell-autonomous Fas (CD95)/Fas ligand interaction mediates activation-induced cell apoptosis of T cell hybridomas. Nature 373:441–444;1995.
Combadiere B, Sousa CR, Trageser C, Zheng L-X, Kim CR, Lenardo MJ. Differential TCR signaling regulates apoptosis and immunopathology during antigen response in vivo. Immunity 9:305–313;1998.
Cutolo M, Sulli A, Seriolo B, Accardo S, Masi AT. Estrogens, the immune response and autoimmunity. Clin Exp Rheum 13:217–226;1995.
Dhein J, Walczak H, Baumler C, Debatin K-M, Krammer PH. Autocrine T-cell suicide mediated by APO-1 (CD95) on T lymphocytes. Nature 373:438–441;1995.
Ettinger R, Panka DJ, Wang JKL, Stanger BZ, Ju S-T, Marshak-Rothstein A. Fas ligand-mediated cytotoxicity is directly responsible for apoptosis of normal CD4+ T cells responding to a bacterial superantigen. J Immunol 154:4302–4308;1995.
Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, Strober W, Lenardo MJ, Puck JM. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 31:935–946;1995.
Fournel S, Genestier L, Robinet E, Flacher M, Revillard JP. Human T cells require IL-2 not G1/S transition to acquire susceptibility to Fasmediated apoptosis. J Immunol 157:4309–4315;1996.
Green DR. Apoptotic pathways: The roads to ruin. Cell 94:695–698;1998.
Heo Y, Lee WT, Lawrence DA. In vivo the environmental pollutants lead and mercury induce oligoclonal T cell responses skewed toward type-2 reactivities. Cell Immunol 179:185–195;1997.
Ju S-T, Cui H, Panka DJ, Ettinger R, Marshak-Rothstein A. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc Natl Acad Sci USA 91:4185–4189;1994.
Ju S-T, Panka DJ, Cui H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 373:444–448;1995.
Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell 49:273–280;1987.
Karandikar N, Vanderlugt Cl, Walunas TL, Miller SD, Bluestone JA. CTLA-4: A negative regulator of autoimmune disease. J Exp Med 184:783–788;1996.
Kawabe Y, Ochi A. Programmed cell death and extrathymic reduction of Vβ8+ CD4+ T cells in mice tolerant toStaphylococcus aureus enterotoxin B. Nature 349:245–247;1991.
Kneitz B, Herrmann T, Yonehara S, Schimpl A. Normal clonal expansion but impaired Fasmediated cell death and anergy induction in interleukin-2-deficient mice. Eur J Immunol 25:2575–2577;1995.
Kroemer G, Hirsch F, Bonzalez-Garcia A, Martinez C. Differential involvement of Th1 and Th2 cytokines in autoimmune diseases. Autoimmunity 24:25–33;1996.
Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4-deficient mice. Science 254:707–710;1993.
Kung JT, Beller D, Ju S-T. Lymphokine regulation of activation-induced apoptosis in T cells of IL-2 and IL-2Rβ knockout mice. Cell Immunol 185;158–163;1998.
Latinis KM, Carr LL, Peterson EJ, Norian LA, Eliason SL, Koretzky GA. Regulation of CD95 (Fas) ligand expression by TCR-mediated signaling events. J Immunol 158:4602–4611;1997.
Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature 353:858–861;1991.
Leonard WJ, Noguchi M, Russell SM, McBride OW. The molecular basis of X-lined severe combined immunodeficiency: The role of interleukin-2 receptor gamma chain as a common γ chain, γc. Immunol Rev 138:61–86;1994.
Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Rev 16:34–38;1995.
Lo D. Animal models of human disease. Transgenic and knockout models of autoimmunity: Building a better disease? Clin Immunol Immunopathol 79:96–104;1996.
Matsui K, Fine A, Zhu B, Marshak-Rothstein A, Ju S-T. Identification of two NF-κB sites in mouse CD95 ligand (Fas ligand) promoter: Functional analysis in T cell hybridoma. J Immunol 161:3469–3473;1998.
Miller RA. The aging immune system: Primer and prospectus. Science 273:70–74;1996.
Mittelstadt PR, Ashwell JD. Cyclosporin A-sensitive transcription factor Egr-3 regulates Fas ligand expression. Mol Cell Biol 18:3744–3751;1998.
Nakajima H, Leonard WJ. Impaired peripheral deletion of activated T cells in mice lacking the common cytokine receptor gamma-chain: Defective Fas ligand expression in gamma-chain-deficient mice. J Immunol 159:4737–4744;1997.
Nelson BH, Wilerford DM. Biology of the interleukin-2 receptor. Adv Immunol 70:1–81;1998.
Ozdemirli M, El-Khatib M, Foote LC, Wang JKM, Marshak-Rothstein A, Rothstein TL, Ju S-T. Fas (CD95)/Fas ligand interactions regulate antigen-specific, major histocompatibility restricted T/B cell proliferative response. Eur J Immunol 26:415–419;1996.
Pahlavani MA, Richardson A. The effect of age on the expression of interleukin-2. Mech Aging Dev 89:125–154;1996.
Peschon JJ, Morrissey PJ, Brabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med 180;1955–1960;1994.
Radvanyi LG, Shi Y, Mills GB, Miller RG. Cell cycle progression out of G1 sensitizes primary-cultured non-transformed T cells to TCR-mediated apoptosis. Cell Immunol 170:260–273;1996.
Radvanyi LG, Mills GB, Miller RG. Religation of the T cell receptor after primary activation of mature T cells inhibits proliferation and induces apoptotic cell death. J Immunol 150:5704–5715;1993.
Ramsdell F, Seaman MS, Miller RE, Picha KS, Kennedy MK, Lynch DH. Differential ability of Th1 and Th2 cells to express Fas ligand and to undergo activation-induced cell death. Int Immunol 6:1545–1553;1994.
Ranger AM, Oukka M, Rengarajan J, Glimcher LH. Inhibitory function of two NFAT family members in lymphoid homeostasis and Th2 development. Immunity 9:627–635;1998.
Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, de Villarty JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 268:1347–1349;1995.
Roths JB, Murphy ED, Eicher EM. A new mutation,gld, that produces lymphoproliferative and autoimmunity in C3H/HeJ mice. J Exp Med 159:1–20;1984.
Rothstein TL, Wang JKM, Panka DJ, Foote LC, Wang Z, Stanger B, Cui H, Ju S-T, Marshak-Rotshein A. Protection against Fas-dependent Th1-mediated apoptosis by antigen receptor engagement in B cells. Nature 374:163–165;1995.
Sadlack B, Kuhn R, Schorle H, Rajewsky K, Muller W, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75:253–261;1993.
Schorle H, Holtschle T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature 352:621–624;1991.
She J, Matsui K, Terhorst C, Ju S-T. Activation-induced apoptosis of mature T cells is dependent upon the level of surface TCR but not on the presence of the CD3 ζ ITAM. Int Immunol 10:1722–1740;1998.
Singer GG, Abbas AK. The Fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity 1:365–371;1994.
Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJL, Ohashi PS, Griesser H, Tanaguchi T, Paige CJ, Mak TW. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor alpha. Science 268:1472–1476;1995.
Takahashi T, Tanaka M, Brannan C, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice caused by a point mutation in the Fas ligand. Cell 76:969–976;1994.
Theofilopoulos AN, Kofler RR, Singer PA, Dixon FJ. Molecular genetics of murine lupus models. Adv Immunol 46:61–109;1989.
Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3:541–547;1995.
Van Parijs L, Biuckians A, Ibragimov A, Alt FW, Willerford DM, Abbas AK. Functional responses and apoptosis of CD25 (IL-2R alpha)-deficient T cells expressing transgenic receptors. J Immunol 158:3738–3745;1997.
Verthelyi DI, Ansar AS. Estrogen increases the number of plasma cells and enhances their autoantibody production in nonautoimmune C57BL/6 mice. Cell Immunol 189:125–134;1998.
Von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SEG, Murray R, Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med 181:1519–1526;1995.
Wang R, Roger AM, Rush BJ, Russell JH. Induction of sensitivity to activation-induced death in primary CD4+ cells: A role for interleukin-2 in the negative regulation of responses by mature CD4+ T cells. Eur J Immunol 26:2263–2270;1996.
Watanabe-Fukugawa R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356:314–317;1992.
Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science 270:985–988;1995.
Willerford DM, Chen J, Ferry JS, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of peripheral lymphoid compartment. Immunity 3:521–530;1995.
Zheng L, Trageser CL, Willerford DM, Lenardo MJ. T cell growth cytokines cause the super-induction of molecules mediating antigen-induced T lymphocyte death. J Immunol 160:763–769;1998.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matsui, K., Jodo, S., Xiao, S. et al. Hypothesis: A recurrent, moderate activation fosters systemic autoimmunity — the apoptotic roles of TCR, IL-2 and fas ligand. J Biomed Sci 6, 306–313 (1999). https://doi.org/10.1007/BF02253519
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02253519