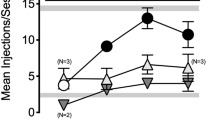
Abstract
Experiments tested the hypothesis that loss of agonist potency or effectiveness following irreversible antagonist or chronic agonist treatment may result from affinity changes at μ opioid receptors. Apparent affinity of naltrexone or nalbuphine for μ opioid receptors was measured in vivo in rats treated with either a single dose of the irreversible antagonist clocinnamox or repeated doses of morphine. Apparent affinity of each antagonist was estimated from its potency as an antagonist of discriminative stimulus or rate-decreasing effects of morphine in rats trained to discriminate 3.2 mg/kg morphine and saline. In control rats, apparent pA2 values for naltrexone and nalbuphine were 7.5–7.6 and 5.3, respectively. In clocinnamox-treated rats, apparent pA2 values for naltrexone were 7.2–7.7, suggesting that clocinnamox treatment did not alter affinity of naltrexone for sites through which morphine exerts behavioral effects. In rats treated repeatedly with morphine, apparent pA2 values for nalbuphine were 5.1–5.3, suggesting that repeated morphine treatment did not alter affinity of nalbuphine for these sites. The observation that neither clocinnamox nor repeated morphine treatment altered in vivo affinity estimates for naltrexone or nalbuphine, respectively, suggests that the reductions in agonist potency produced by these treatments do not result from changes in affinity at μ opioid receptors.
Similar content being viewed by others
References
Adams JU, Paronis CA, Holtzman SG (1990) Assessment of relative intrinsic activity of μ-opioid analgesic in vivo by using β-funaltrexamine. J Pharmacol Exp Ther 255: 1027–1032
Arunlakshana O, Schild H (1959) Some quantitative uses of drug antagonists. Br J Pharmacol 14: 48–58
Burke TF, Woods JH, Lewis JW, Medzihradsky F (1994) Irreversible opioid antagonist effects of clocinnamox on opioid analgesics andmu receptor binding in mice. J Pharmacol Exp Ther 271: 650–661
Comer SD, Burke TF, Lewis JW, Woods JH (1992) Clocinnamox: a novel, systemically-active, irreversible opioid antagonist. J Pharmacol Exp Ther 262[3]: 1051–1056
Dum J, Blasig J, Meyer G, Herz A (1979)In vivo opiate binding unchanged in tolerant/dependent mice. Eur J Pharmacol 55: 375–383
Dykstra LA (1990) Butorphanol, levallorphan, nalbuphine and nalorphine as antagonists in the squirrel monkey. J Pharmacol Exp Ther 254[1]: 245–252
Fernandes M, Kluwe S, Coper H (1977a) The development of tolerance to morphine in the rat. Psychopharmacology 54: 197–201
Fernandes M, Kluwe S, Coper H (1977b) Quantitative assessment of tolerance to and dependence on morphine in mice. Naunyn-Schmiedeberg's Arch Pharmacol 297: 53–60
France CP, Woods JH (1987) β-Funaltrexamine antagonizes the discriminative stimulus effects of morphine but not naltrexone in pigeons. Psychopharmacology 91: 213–216
France CP, DeCosta BR, Jacobson AE, Rice KC, Woods JH (1990) Apparent affinity of opioid antagonists in morphine-treated rhesus monkeys discriminating between saline and naltrexone. J Pharmacol Exp Ther 252[2]: 600–604
Gerak LR, Butelman ER, Woods JH, France CP (1994) Antinociceptive and respiratory effects of nalbuphine in rhesus monkeys. J Pharmacol Exp Ther 271[2]: 993–999
Harris R, Loh HH, Way E (1976) Alterations in the efficacy of naloxone induced by stress, cyclic adenosine monophosphate, and morphine tolerance. Eur J Pharmacol 39: 1–10
Hollt, V, Dum, J, Blasig, J, Schubert, P, Herz, A (1975) Comparison of in vivo and in vitro parameters of opiate receptor binding in naive and tolerant/dependent rodents. Life Sci 16: 1823–1828
Holtzman SG (1983) Discriminative stimulus properties of opioid agonists and antagonists. In: Cooper S (ed) Theory in psychopharmacology. Academic Press, London, pp 1–45
Jaffe JH, Martin W (1985) Opioid analgesics and antagonists. In: Gilman AG, Goodman LS, Rall TW, Murad F (eds) The pharmacological basis of therapeutics. MacMillan, New York
Kenakin T (1993) Pharmacologic analysis of drug-receptor interaction. Raven Press, New York
Mucha RF, Kalant H (1980) Log dose/response curve flattening in rats after daily injections of opiates. Psychopharmacology 71: 51–61
Paronis CA, Holtzman SG (1992) Development of tolerance to the analgesic activity of μ agonists following continuous infusion of morphine, meperidine, or fentanyl in rats. J Pharmacol Exp Ther 262: 1–9
Picker MJ, Yarbrough J, Hughes CE, Smith MA, Morgan D, Dykstra LA (1993) Agonist and antagonist effects of mixed action opioids in the pigeon drug discrimination procedure: influence of training dose, intrinsic efficacy and interanimal differences. J Pharmacol Exp Ther 266[2]: 756–767
Takemori A (1974) Determination of pharmacological constants: use of narcotic antagonists to characterize analgesic receptors. In: Braude M, Harris L, May E, Smith J, Villarreal J (eds) Narcotic antagonists. Advances in biochemical psychopharmacology, vol. 8. Raven Press, New York, pp 335–344
Takemori A, Portoghese P (1984) Comparative antagonism of mu, kappa, and delta agonists. Eur J Pharmacol 104: 101–104
Tallarida, RJ, Murray, RB (1987) Manual of pharmacologic calculations with computer programs, 2nd edn. Springer, New York
Teal JJ, Holtzman SG (1980) Stimulus effects of morphine in the monkey: quantitative analysis of antagonism. Pharmacol Biochem Behav 12: 587–593
Tulunay F, Takemori A (1974) Further studies on alteration of analgesic receptor-antagonist interaction induced by morphine. J Pharmacol Exp Ther 190: 401–407
Walker EA, Butelman ER, DeCosta BR, Woods JH (1993) Opioid thermal antinociception in rhesus monkeys: receptor mechanisms and temperature dependency. J Pharmacol Exp Ther 267[1]: 280–286
Walker EA, Makhay MM, House JD, Young AM (1994) In vivo apparent pA2 analysis for naltrexone antagonism of discriminative stimulus and analgesic effects of opiate agonists in rats. J Pharmacol Exp Ther 271[2]: 959–968
Young AM, Kapitsopoulos G, Makhay M (1991) Tolerance to morphine-like stimulus effects ofmu opioid agonists. J Pharmacol Exp Ther 257: 795–805
Young AM, Masaki MA, Geula C (1992) Discriminative stimulus effects of morphine: effects of training dose on agonist and antagonist effects ofmu opioids. J Pharmacol Exp Ther 261[1]: 246–257
Zernig G, Broadbear J, Burke T, Lewis JW, Brine GA, Woods JH (1995) Receptor reserve and affinity of μ opioid agonists in mouse antinociception: correlation with receptor binding. Life Sci 57: 2113–2125
Author information
Authors and Affiliations
Additional information
This work was supported by US Public Health Service Grant DA03796 and NIDA Research Scientist Development Award K02 DA00132 to A.M. Young. A portion of this work is presented in abstract form [Walker EA, Richardson TM, Young AM (1995) Apparent affinity estimates for opioid antagonists in rats treated with clocinnamox or chronic morphine. In: Harris LS (ed) NIDA Res Monogr 153: 450]
Rights and permissions
About this article
Cite this article
Walker, E.A., Richardson, T.M. & Young, A.M. In vivo apparent pA2 analysis in rats treated with either clocinnamox or morphine. Psychopharmacology 125, 113–119 (1996). https://doi.org/10.1007/BF02249409
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02249409