Abstract
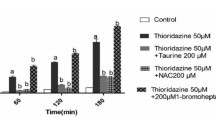
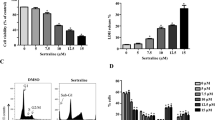
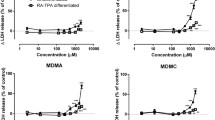
Recent interest in the neurotoxicity of haloperidol is based on its oxidation in rodents to the pyridinium derivative, HPP+, a structural analog of the neurotoxin, 1-methyl-4-phenylpyridinium (MPP+). Recently, we reported that HPP+ and a newly identified reduced pyridinium, RHPP+, were present in blood and urine of haloperidol-treated schizophrenics and that the concentrations of RHPP+ exceeded those of HPP+. In this study, we examined pathways for formation of RHPP+ in subcellular fractions of human liver (n=5) and brain (basal ganglia;n=5). The major pathway was reduction of HPP+ (20 µM) to RHPP+ in cytosol (0.17–0.39 and 0.03–0.07 µM RHPP+/g cytosolic protein per h in liver and brain, respectively). The reactions were inhibited significantly by menadione and in brain also by daunorubicin. The inhibition profile, cytosolic location and strict NADPH dependence suggest that the enzymes involved are ketone reductases. A second pathway was oxidation of reduced haloperidol (50 µM), a major metabolite of haloperidol in blood and brain, to RHPP+. In liver microsomes, 0.17–0.63 µmol RHPP+ was formed /g microsomal protein per h. A potent inhibitor of the pathway was ketoconazole (IC50, 0.8 µM), which suggests that P-450 3A isozymes could be involved. In brain mitochondria but not microsomes, reduced haloperidol (120 µM) was oxidised to RHPP+ at a small but significant rate (0.005–0.020 µmol RHPP+/g mitochondrial protein per h) which was not attenuated by SKF 525A, quinidine, ketoconazole, or monoamine oxidase inhibitors. Further studies are warranted to establish the biological importance of these metabolites in vivo.
Similar content being viewed by others
References
Bachur NR (1976) Cytoplasmic aldo-keto reductases: a class of drug metabolising enzymes. Science 193:595–597
Bloomquist J, King E, Wright A, Mytilineou C, Kimura K, Castagnoli K, Castagnoli Jr N (1994) 1-Methyl-4-phenylpyridinium-like neurotoxicity of a pyridinium metabolite derived from haloperidol: cell culture and neurotransmitter uptake studies. J Pharmacol Exp Ther 270:822–830
Bocker RH, Guengerich FP (1986) Oxidation of 4-aryl- and 4-alkyl-substituted 2,6-dimethly-3,5-bis(alkoxycarbonyl)-1,4-dihydropyridines by human liver microsomes and immunochemical evidence for the involvement of a form of cytochrome P-450. J Med Chem 29:1596–1603
Eyles DW, Pond SM (1992) Stereospecific reduction of haloperidol in human tissues. Biochem Pharmacol 44:867–871
Eyles DW, McLennan HR, Jones A, McGrath JJ, Stedman TJ, Pond SM (1994a) Quantitative analysis of two pyridinium metabolites of haloperidol in patients with schizophrenia. Clin Pharmacol Ther 56:512–520
Eyles DW, Stedman TJ, Pond SM (1994b) Nonlinear relationship between circulating concentrations of reduced haloperidol and haloperidol: evaluation of possible mechanisms. Psychopharmacology 116:161–166
Fang J, Gorrod JW (1993) High performance liquid chromatographic method for the detection and quantitation of haloperidol and seven of its metabolites in microsomal preparations. J Chromatogr 614:267–273
Forsman A, Folsch G, Larsson M, Ohman R (1977) On the metabolism of haloperidol in man. Curr Ther Res 21:606–617
Ghersi-egea J-F, Perrin R, Leininger-Muller B, Grassiot M-C, Jeandel C, Floquet J, Cuny G, Siest G, Minn A (1993) Subcellular location of cytochrome P450, and activities of several enzymes responsible for drug metabolism in the human brain. Biochem Pharmacol 45:647–658
Gorrod JW, Fang J (1993) On the metabolism of haloperidol. Xenobiotica 23:495–508
Guengerich FP (1990) Mechanism-based inactivation of liver microsomal cytochrome P-450 IIIA4 by gestodene. Chem Res Toxicol 3:363–371
Halpert JR, Guengerich FP, Bend JR, Correia MA (1994) Selective inhibitors of cytochromes P450. Toxicol Appl Pharmacol 125:163–175
Igarashi K, Castagnoli Jr N (1992) Determination of the pyridinium metabolite derived from haloperidol in brain tissue, plasma and urine by high-performance liquid chromatography with fluorescence detection. J Chromatogr 579:277–283
Korpi ER, Kleinman JE, Costakos DT, Linnoila M, Wyatt RJ (1984) Reduced haloperidol in the post-mortem brains of haloperidol-treated patients. Psychiatry Res 11:259–269
Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219:979–980
Lerena AL, Alm C, Dahl M-L, Ekqvist B, Bertilsson L (1992) Haloperidol disposition is dependent on debrisoquine hydroxylation phenotype. Ther Drug Monit 14:92–97
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Oida T, Terauchi Y, Yoshida K, Kagemoto A, Sekine Y (1989) Use of antisera in the isolation of human specific conjugates of haloperidol. Xenobiotica 19:781–793
Rollema H, Skolnik M, D'Engelbronner J, Igarashi K, Usuki E, Castagnoli Jr N (1994) MPP+-like neurotoxicity of a pyridinium metabolite derived from haloperidol: in vivo microdialysis and in vitro mitochondrial studies. J Pharmacol Exp Ther 268:380–387
Someya T, Shibasaki M, Noguchi T, Takahashi S, Inaba T (1992) Haloperidol metabolism in psychiatric patients: importance of glucuronidation and carbonyl reduction. J Clin Psychopharmacol 12:169–174
Spina E, Ancione M, Di Rosa AE, Meduri M, Caputi AP (1992) Polymorphic debrisoquine oxidation and acute neuroleptic-induced adverse effects. Eur J Clin Pharmacol 42:347–348
Stedman TJ, Whiteford HA Eyles DW, Welham JL, Pond SM (1991) Effects of nifedipine on psychosis and tardive dyskinesia in schizophrenic patients. J Clin Psychopharmacl 11:43–47
Subramanyam B, Rollema H, Woolf T, Castagnoli Jr N (1990) Identification of a potentially neurotoxic pyridinium metabolite of haloperidol in rats. Biochem Biophys Res Commun 166:238–244
Subramanyam B, Woolf T, Castagnoli Jr N (1991) Studies on the in vitro conversion of haloperidol to a potentially neurotoxic pyridinium metabolite. Chem Res Toxicol 4:123–128
Tipton KF, Singer TP (1993) Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J Neurochem 61:1191–1206
Van der Schyf CJ, Castagnoli K, Usuki E, Fouda HG, Rimoldi JM, Castagnoli Jr N (1994) Metabolic studies of haloperidol and its tetrahydropyridine analog in C57BL/6 mice. Chem Res Toxicol 7:281–285
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eyles, D.W., McGrath, J.J. & Pond, S.M. Formation of pyridinium species of haloperidol in human liver and brain. Psychopharmacology 125, 214–219 (1996). https://doi.org/10.1007/BF02247331
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02247331