Abstract
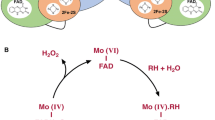
Monoamine oxidases A and B (MAO-A and B) catalyze the oxidative catabolism of biogenic amines and xenobiotics. Investigation of these mitochondrial membrane proteins shows that they differ in substrate preference, inhibitor specificity, tissue and neuronal cell distribution, immunological properties, and nucleotide and deduced amino acid sequences. Comparisons of MAO-A and B from the human, bovine, and rat species show strikingly high similarity (85–88%) in the amino acid sequences of each enzyme. Furthermore, three regions in MAO-A and B have sequence identities across species of 78, 88, and 86%. These regions correspond to a nucleotide-binding site near the N-terminal end that is found in the vast majority of enzymes that require flavin adenine dinucleotide (FAD), a region of unknown function, and the FAD-binding site toward the C-terminal end. Genomic clones of MAO-B which span almost the entire gene (>40 kb) have been isolated, restriction mapped, and partially sequenced. Likewise, genomic clones of MAO-A that correspond to the 3′-flanking region have also been investigated. Current studies which focus on identification of the promotor and regulatory sequences should help to establish why MAO-A and B are localized in different subsets of neurons in brain.
Similar content being viewed by others
References
Bach AWJ, Lan NC, Johnson DL, Abell CW, Bembenek ME, Kwan S-W, Seeburg PH, Shih JC (1988) cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc Natl Acad Sci USA 85:4934–4938
Benton WD, Davis PW (1977) Screening λgt recombinant clones by hybridization to single plaques in situ. Science 196:180–182
Berrettini WH, Benfield TC, Schmidt AO, Ladman RK, Vogel WH (1980) Platelet monoamine oxidase in families of chronic schizophrenics. Schizophr Bull 6:235–237
Bond PA, Cundall RL (1977) Properties of monoamine oxidase (MAO) in human blood platelets, plasma, lymphocytes and granulocytes. Clin Chim Acta 80:317–325
Chiba K, Trevor A, Castagnoli Jr. N (1984) Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem Biophys Res Commun 120:574–578
Chuang HYK, Patek DR, Hellerman L (1974) Mitochondrial monoamine oxidase: inactivation by pargyline, adduct formation. J Biol Chem 249:2381–2384
Cloninger CR, von Knorring L, Oreland L (1985) Pentametric distribution of platelet monoamine oxidase activity. Psychiatry Res 15:133–143
DaPrada M, Kettler R, Keller HH, Burkard WP, Muggli-Maniglio D, Haefely W (1989) Neurochemical profile of moclobemide, a short-acting and reversible inhibitor of monoamine oxidase type A. J Pharmacol Exp Ther 248:400–414
Davis GC, Williams AC, Markey SP, Ebert MH, Caine ED, Reichert CM, Kopin IJ (1979) Chronic parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res 1:249–254
Denney RM, Fritz RR, Patel NT, Abell CW (1982) MAO A and B from human liver separated by immunoaffinity column chromatography using an MAO B-specific monoclonal antibody. Science 215:1400–1403
Denney RM, Dave S, Sharma A (1990) Isolation of sequences at or near the 5′ ends of the human MAO A and B genes. Soc Neurosci Abstr 16:662
Donnelly CH, Murphy DL (1977) Substrate- and inhibitor-related characteristics of human platelet monoamine oxidase. Biochem Pharmacol 26:853–858
Egashira T (1976) Enzymic properties of placental monoamine oxidase. Jpn J Pharmacol 26:493–500
Feinberg AP, Vogelstein B (1983) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132:6–13
Fritz RR, Abell CW, Denney RM, Denney CB, Bessman JD, Boeringa JA, Castellani S, Lankford DA, Malek-Ahmadi P, Rose RM (1985) Platelet MAO concentration and molecular activity: I. New methods using an MAO B-specific monoclonal antibody in a radioimmunoassay. Psychiatry Res 17:129–140
Goate A, Chartier-Harlin M-C, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, Mantl R, Newton P, Rooke K, Roques P, Talbot C, Pericak-Vance M, Roses A, Williamson R, Rossor M, Owen M, Hardy J (1991) Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 349:704–706
Greenwalt JW, Schnaitman C (1970) An appraisal of the use of monoamine oxidase as an enzyme marker for the outer membrane of rat liver mitochondria. J Cell Biol 46:173–179
Grimsby J, Chen K, Lan N, Shih JC (1990) The genomic organization of human MAO B gene. Soc Neurosci Abstr 16:662
Hsu Y-PP, Weyler W, Chen S, Sims KB, Rinehart WB, Utterback M, Powell JF, Breakefield XO (1988) Structural features of human monoamine oxidase A elucidated from cDNA and peptide sequences. J Neurochem 51:1321–1324
Ito A, Kuwahara T, Inadome S, Sagara Y (1988) Molecular cloning of a cDNA for rat liver monoamine oxidase B. Biochem Biophys Res Commun 157:970–976
Johnston JP (1968) Some observations upon a new inhibitor of monoamine oxidase in human brain. Biochem Pharmacol 17:1285–1297
Kearney EB, Salach JI, Walker WH, Seng RL, Kenney W, Zeszotek E, Singer TP (1971) The covalently-bound flavin of hepatic monoamine oxidase: Isolation and sequence of a flavin peptide and evidence for binding at the 8-alpha position. Eur J Biochem 24:321–327
Kochersperger LM, Waguespack A, Patterson JC, Hsieh CCW, Weyler W, Salach JI, Denney RM (1985) Immunological uniqueness of human monoamine oxidases A and B: new evidence from studies with monoclonal antibodies to human MAO-A. J Neurosci 11:2874–2881
Kochersperger LM, Parker EL, Siciliano M, Darlington GJ, Denney RM (1986) Assignment of genes for human monoamine oxidases A and B to the X chromosome. J Neurosci Res 16:601–616
Kuwahara T, Takamoto S, Ito A (1990) Primary structure of rat monoamine oxidase deduced from cDNA and its expression in rat tissues. Agric Biol Chem 54:253–257
Lan NC, Chen C, Shih JC (1989a) Expression of functional human monoamine oxidase A and B cDNAs in mammalian cells. J Neurochem 52:1652–1654
Lan NC, Heinzmann C, Gal A, Klisak I, Orth U, Lai E, Grimsby J, Sparkes RS, Mohandas T, Shih JC (1989b) Human monoamine oxidase A and B genes map to Xp11.23 and are deleted in a patient with Norrie disease. Genomics 4:552–559
Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic parkinsonism in humans due to a product of meperidine-analog syntheses. Science 219:979–980
Levitt P, Pintar JE, Breakefield XO (1982) Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad Sci USA 79:6385–6389
Levy ER, Powell JF, Buckle VJ, Hsu Y-PP, Breakefield XO, Craig IW (1989) Localization of human monoamine oxidase-A gene to Xp11.23-11.4 by in situ hybridization: implications for Norrie disease. Genomics 5:368–370
Minamiura N, Yasunobu KT (1978) Bovine liver monoamine oxidase: a modified purification procedure and preliminary evidence for two subunits and one FAD. Arch Biochem Biophys 189:481–489
Murphy DL, Belmaker R, Wyatt RJ (1974) Monoamine oxidase in schizophrenia and other behavioral disorders. J Psychiatr Res 11:221–247
Ozelius L, Hsu Y-PP, Bruns G, Powell JF, Chen S, Weyler W, Utterback M, Zucker D, Haines J, Trofatter JA, Conneally PM, Gusella JF, Breakefield XO (1988) Human monoamine oxidase gene (MAO A): chromosome position (Xp21-p11) and DNA polymorphism. Genomics 3:53–58
Pintar JE, Barbosa J, Francke U, Castiglione CM, Hawkins Jr. M, Breakefield XO (1981) Gene for monoamine oxidase type A assigned to the human X chromsome. J Neurosci 1:166–175
Powell JF, Hsu Y-PP, Weyler W, Chen S, Salach JI, Andrikopoulos K, Mallet J, Breakefield XO (1989) The primary structure of bovine monoamine oxidase type A. Biochem J 259:407–413
Reveley MA, Reveley AM, Clifford CA, Murray RM (1983) Genetics of platelet MAO activity in discordant schizophrenic and normal twins. Br J Psychiatry 142:560–565
Riley LA, Waguespack MA, Denney RM (1989) Characterization and quantitation of monoamine oxidases A and B in mitochondria from human placenta. Mol Pharmacol 36:54–60
Rose RM, Castellani S, Boeringa JA, Malek-Ahmadi P, Lankford DA, Bessman JD, Fritz RR, Denney CB, Denney RM, Abell CW (1986) Platelet MAO concentration and molecular activity: II. Comparison of normal and schizophrenic populations. Psychiatry Res 17:141–151
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Sandler M, Reveley MA, Glover V (1981) Human platelet monoamine oxidase activity in health and disease: a review. J Clin Pathol 34:292–302
von Korff RW (1979) Monoamine oxidase: unanswered questions. In: Singer TP, von Korff RW, Murphy DL (eds) Monoamine oxidase: structure, function and altered functions. Academic Press, New York, pp 1–7
Westlund KN, Denney RM, Kochersperger LM, Rose RM, Abell CW (1985) Distinct monoamine oxidase A and B populations in primate brain. Science 230:181–183
Westlund KN, Denney RM, Rose RM, Abell CW (1988) Localization of distinct monoamine oxidase A and monoamine oxidase B cell populations in human brain stem. Neuroscience 25:439–456
Weyler W (1989) Monoamine oxidase A from human placenta and monoamine oxidase B from bovine liver both have one FAD per subunit. Biochem J 260:725–729
White HL, Tansik RL (1979) Characterization of multiple substrate binding sites of MAO. In: Singer TP, von Korff RW, Murphy DL (eds) Monoamine oxidase: structure, function, and altered functions. Academic Press, New York, pp 129–144
Wyatt RJ, Potkin SG, Bridge TO, Phelps BH, Wise CD (1980) Monoamine oxidase in schizophrenia: an overview. Schizophr Bull 6:199–207
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kwan, SW., Bergeron, J.M. & Abell, C.W. Molecular properties of monoamine oxidases A and B. Psychopharmacology 106 (Suppl 1), S1–S5 (1992). https://doi.org/10.1007/BF02246224
Issue Date:
DOI: https://doi.org/10.1007/BF02246224