Abstract
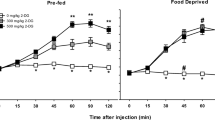
An important aspect of motivated behavior is that organisms will perform complex instrumental behaviors to gain access to stimuli such as food. In the present study, food-deprived rats were tested in an operant chamber in which the animals had a choice between pressing a lever to obtain a more-preferred food (Bioserve pellets), or free feeding on a less-preferred food (lab chow). Typically, rats pressed the lever to obtain the preferred food pellets, and ate little of the less-preferred food even though it was freely available. Pre-fed rats showed suppression of both lever pressing and feeding. Systemic administration of 0.1 mg/kg haloperidol (HP) led to a dramatic shift in the behavior of these rats, such that the number of lever presses was substantially reduced, but the amount of less-preferred food consumed showed a significant increase. This result occurred if the rats pressed a lever on either a CRF or FR5 schedule. Injection of 3.5–7.0 µg HP directly into the nucleus accumbens, or intra-accumbens injections of 6-hydroxy-dopamine, also decreased lever pressing for food and increased feeding on laboratory chow. Thus, interference with brain dopamine suppressed a highly active instrumental response for food, although the behavior of the animal was still directed towards food acquisition and consumption.
Similar content being viewed by others
References
Asin KE, Fibiger HC (1984) Force requirements in lever-pressing and responding after haloperidol. Pharmacol Biochem Behav 20:323–326
Beninger RJ (1983) The role of dopamine activity in locomotor activity and learning. Brain Res Rev 6:173–196
Beninger RJ, Phillips AG (1980) The effect of pimozide on the establishment of conditioned reinforcement. Psychopharmacology 68:147–153
Berridge KC, Venier IL, Robinson TE (1989) Taste reactivity analysis of 6-hydroxydopamine-induced aphagia: implications for arousal and anhedonia hypotheses of dopamine function. Behav Neurosci 103:36–45
Blackburn JR, Phillips AG, Fibiger HC (1987) Dopamine and preparatory behavior. I: Effects of pimozide. Behav Neurosci 101:352–360
Blackburn JR, Phillips AG, Fibiger HC (1989a) Dopamine and preparatory behavior. III: Effects of metoclopramide and thioridazine. Behav Neurosci 103:903–906
Blackburn JR, Phillips AG, Jakubovic A, Fibiger HC (1989b) Dopamine and preparatory behavior. III: A neurochemical analysis. Behav Neurosci 103:15–23
Bowers W, Hamilton M, Zacharcho RM, Anisman H (1985) Differential effects of pimozide on response-rate and choice accuracy in a self-stimulation paradigm in mice. Pharmacol Biochem Behav 22:521–526
Cador M, Robbins TW, Everitt BJ (1989) Involvement of the amygdala in stimulus-reward associations: interactions with the ventral striatum. Neuroscience 30:79–84
Church WH, Justice JB, Neill DB (1987) Detecting behaviorally relevant changes in extracellular dopamine with microdialysis. Brain Res 412:397–399
Ettenberg A, Koob GF, Bloom F (1981) Response artifact in the measurement of neuroleptic-induced anhedonia. Science 209:357–359
Evenden JL, Robbins TW (1983) Dissociable effects ofd-amphetamine, chlordiazepoxide and alpha-flupenthixol on choice and rate measures of reinforcement in the rat. Psychopharmacology 79:180–186
Everitt BJ (1990) Sexual motivation: a neural and behavioral analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev 14:217–232
Everitt BJ, Cador M, Robbins TW (1989) Interactions between the amygdala and ventral striatum in stimulus-reward associations: studies using a second-order schedule of sexual reinforcement. Neuroscience 30:63–75
Faustman WO, Fowler SC (1981) Use of operant response duration to distinguish effects of haloperidol from non-reward. Pharmacol Biochem Behav 15:327–329
Faustman WO, Fowler SC (1982) An examination of methodological refinements, clozapine and fluphenazine in the anhedonia paradigm. Pharmacol Biochem Behav 17:987–993
Fibiger HC, Carter DA, Phillips AG (1976) Decreased intracranial self-stimulation after neuroleptics or 6-hydroxydopamine: evidence for mediation by motor deficits rather than by reduced reward. Psychopharmacology 47:21–27
Horvitz JC, Ettenberg A (1989) Haloperidol blocks the response-reinstating effects of food reward: a methodology for separating neuroleptic effects on reinforcement and motor processes. Pharmacol Biochem Behav 31:861–865
Hursh SR, Raslear TG, Shurtleff D, Bauman R, Simmons L (1988) A cost-benefit analysis of demand for food. J Exp Anal Behav 50:419–440
Iversen SD (1977) Brain dopamine systems and behavior. In: Iversen LL, Iversen SD, Snyder SH (eds) Handbook of psychopharmacology. Plenum Press, New York, pp 333–384
Janssen PA, Niemegeers CJF, Schellekens KHL (1965) Is it possible to predict the clinical effects of neuroleptic drugs (major tranquilizers) from animal data? Arzneimittelforschung 15:104–117
Kaufman LW (1980) Foraging costs and meal patterns in ferrets. Physiol Behav 25:139–141
Keefe KA, Salamone JD, Zigmond MJ, Stricker EM (1989) Paradoxical kinesia in Parkinsonism is not caused by dopamine release: Studies in an animal model. Arch Neurol 46:1070–1075
Kelley AE, Stinus L (1985) Disappearance of hoarding behavior after 6-hydroxydopamine lesions of the mesolimbic dopamine neurons and its reinstatement with L-DOPA. Behav Neurosci 99:531–5435
Kelly PH, Seviour PW, Iversen SD (1975) Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res 94:507–522
Koob GF, Riley SJ, Smith SC, Robbins TW (1978) Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. J Comp Physiol Psychol 92:917–927
Krebs JR (1978) Optimal foraging: Decision rules for predators. In: Krebs JR, Davies WB (eds) Behavioral ecology. Sinauer Associates, Sunderland, MA
Ljungberg T (1987) Blockade by neuroleptics of water intake and operant responding in the rat: anhedonia, motor deficit or both? Pharmacol Biochem Behav 27:341–350
Ljungberg T (1988) Scopolamine reverses haloperidol-attenuated lever pressing for water but not haloperidol-attenuated water intake in the rat. Pharmacol Biochem Behav 29:205–208
Ljungberg T (1989) Effects of the dopamine D-1 antagonist SCH 23390 on water intake, water-rewarded operant responding and apomorphine induced decrease of water in rats. Pharmacol Biochem Behav 33:709–712
Ljungberg T (1990) Differential attenuation of water intake and water-rewarded operant responding by repeated administration of haloperidol and SCH 23390 in the rat. Pharmacol Biochem Behav 35:111–115
Marshall JF, Richardson JS, Teitelbaum P (1974) Nigrostriatal damage and the lateral hypothalamic syndrome. J Comp Physiol Psychol 87:808–830
Mason ST, Beninger RJ, Fibiger HC, Phillips AG (1980) Pimozide-induced suppression of responding: evidence against a block of food reward. Pharmacol Biochem Behav 12:917–923
McCullough LD, Singer D, Salamone JD (1990) Periodic food presentation increases dopamine and metabolites in nucleus accumbens dialysis perfusates. Soc Neurosci Abstr 16[1]:438
McDowell JJ, Kessell R (1979) A multivariate rate equation for variable-interval performance. J Exp Anal Behav 31:267–283
Mogenson G, Jones D, Yim CY (1980) From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14:69–97
Muscat R, Willner P (1989) Effects of dopamine receptor antagonists on sucrose consumption and preference. Psychopharmacology 99:98–102
Neill DB, Justice JB (1981) An hypothesis for a behavioral function of dopaminergic transmission in nucleus accumbens. In: Chronister RB, Defrance JF (eds) The neurobiology of the nucleus accumbens. Huer Institute, Brunswick
Posluns D (1962) An analysis of chlorpromazine-induced suppression of the avoidance response. Psychopharmacology 3:361–373
Rachlin H (1981) Absolute and relative consumption space. In: Bernstein D (ed) Response structure and organization. University of Nebraska Press, Lincoln
Robbins TW, Everitt BJ (1982) Functional studies of central catecholamines. Int Rev Neurobiol 23:303–364
Rolls ET, Rolls BJ, Kelly PH, Shaw SG, Wood RJ, Dale R (1974) The relative attenuation of self-stimulation, eating and drinking produced by dopamine-receptor blockade. Psychopharmacology 38:219–230
Salamone JD (1986) Different effects of haloperidol and extinction on instrumental behaviors. Psychopharmacology 88:18–23
Salamone JD (1987) The actions of neuroleptic drugs on appetitive instrumental behaviors. In: Iversen LL, Iversen SD, Snyder SH (eds) Handbook of psychopharmacology. Plenum Press, New York
Salamone JD (1988) Dopaminergic involvement in activational aspects of motivation: effects of haloperidol on schedule-induced activity, feeding and foraging in rats. Psychobiology 16:196–206
Salamone JD (1991) Behavioral pharmacology of dopamine systems: a new synthesis. In: Willner P, Scheel-Kruger J (eds) The mesolimbic dopamine system: from motivation to action. John Wiley, Chichester, England, pp 599–613
Salamone JD, Keller RW, Zigmond MJ, Stricker EM (1989) Behavioral activation in rats increases striatal dopamine metabolism measured by dialysis perfusion. Brain Res 487:215–224
Salamone JD, Zigmond MJ, Stricker EM (1990) Characterization of the impaired feeding behavior in rats given haloperidol or dopamine-depleting brain lesions. Neuroscience 39:17–24
Staddon JE (1979) Operant behavior as adaptation to constraint. J Exp Psychol Gen 108:48–67
Stellar JR, Kelley AE, Corbett D (1983) Effects of peripheral and central dopamine blockade on lateral hypothalamic self-stimulation: evidence for both reward and motor deficits. Pharmacol Biochem Behav 18:433–442
Stricker EM, Zigmond MJ (1976) Recovery of function after damage to central catecholamine-containing neurons: a neuro-chemical model for the lateral hypothalamic syndrome. In: Sprague JM (ed) Progress in psychobiology and physiological psychology. Academic Press, New York, pp 121–173
Taylor JR, Robbins TW (1984) Enhanced behavioral control by conditioned reinforcers following microinjections ofd-amphetamine into the nucleus accumbens. Psychopharmacology 84:405–412
Taylor JR, Robbins TW (1986) 6-Hydroxydopamine lesions of the nucleus accumbens but not the caudate nucleus attenuate responding with reward-related stimului produced by intra-accumbensd-amphetamine. Psychopharmacology 90:390–397
Tombaugh TN, Anisman H, Tombaugh J (1980) Extinction and dopamine receptor blockade after intermittent reinforcement training: failure to observe functional equivalence. Psychopharmacology 70:19–28
Tombaugh TN, Szostak C, Mills P (1983) Failure of pimozide to disrupt acquisition of light-dark and spatial discrimination problems. Psychopharmacology 79:161–168
Towell A, Muscat R, Willner P (1987) Effects of pimozide on sucrose consumption and preference. Psychopharmacology 92:262–264
Ungerstedt U (1971) Adipsia and aphagia after 6-hydroxydopamine-induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand 83 [suppl 367]:95–122
Willner P, Chawala K, Sampson D, Sophokleous S, Muscat R (1988) Tests of functional equivalence between pimozide pretreatment, extinction and free feeding. Psychopharmacology 95:423–426
Wise RA (1982) Neuroleptics and operant behavior: the anhedonia hypothesis. Behav Brain Sci 5:39–87
Wise RA (1985) The anhedonia hypothesis: Mark III. Behav Brain Sci 8:178–184
Wise RA (1988) Psychomotor stimulant properties of addictive drugs. Ann NY Acad Sci 537:228–234
Wise RA, Spindler J, DeWitt H, Gerber GJ (1978a) Pimozide blocks reward quality of food. Science 201:262–264
Wise RA, Spindler J, Legault L (1978b) Major attenuation of food reward with performance-sparing doses of pimozide in the rat. Can J Psychol 32:77–85
Zigmond MJ, Stricker EM (1985) Adaptive properties of monoaminergic neurons. In: Lajtha J (ed) Handbook of neurochemistry. Plenum Press, New York, pp 87–102
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Salamone, J.D., Steinpreis, R.E., McCullough, L.D. et al. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology 104, 515–521 (1991). https://doi.org/10.1007/BF02245659
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02245659