Abstract
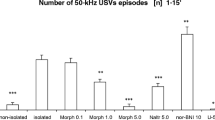
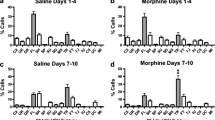
Ultrasonic vocalizations may be an expression of the affective pain response in laboratory animals. The present experiment compares the effects of morphine to the delta agonist, DPDPE (d-Pen2,d-Pen5 enkephalin) on a range of reflexive, behavioral and affective responses during an aggressive interaction. In experiment 1, naive female Long-Evans rats received morphine (0, 1, 3, 6, 10 µg ICV), or DPDPE (0, 30, 60, 100 µg ICV). In experiment 2, female rats were treated with naltrindole (1.0 mg/kg IP) 20 min before DPDPE (0, 60, 100 µg ICV). The following endpoints were measured: (1) latency to tail flick in response to heat stimuli; (2) high (33–65 kHz) and low (20–32 kHz) frequency ultrasonic and audible vocalizations; (3) defensive behavior; and (4) motoric activity. Following a brief exposure to attack, rats were threatened by the aggressor but protected from further attack by a large, wire mesh cage, thereby allowing for continued behavioral and vocal measurement without the risk of physical injury; video and audio recordings were made during the attack and then during a portion of the protected encounter (2 min). Morphine suppressed pain reactions varying in complexity from a spinal reflex, to an organized escape reaction, to an affective vocal response. The delta agonist, DPDPE, attenuated high frequency ultrasonic calling and tail flick responding. Defensive behaviors were also modulated by DPDPE at doses that had no effect on walking or rearing, indicating behavioral specificity. By contrast, doses of morphine that decreased defensive upright and escape also decreased motor activity. In female rats, morphine and DPDPE share a common profile of effects on a range of functional end-points, but DPDPE appears to modulate more selectively the reactions related to aversiveness without exerting sedative effects. These data demonstrate a possible physiological role for delta receptors in affective and defensive reactions.
Similar content being viewed by others
References
Aubin T, Bremond JC (1992) Perception of distress call harmonic structure by the starling (Sturnus vulgaris). Behaviour 120:151–163
Bornschein RL, Crockett RS, Smith RP (1977) Diurnal variations in the analgesic effectiveness of morphine in mice. Pharmacol Biochem Behav 6:621–626
Calcagnetti DJ, Fanselow MS, Helmstetter FJ, Bowen WD (1989) [d-Ala2, Leu5, Cys6] Enkephalin: short-term agonist effects and long-term antagonism at delta opioid receptors. Peptides 10:319–326
Cowan A, Zhu XZ, Mosberg HI, Omnaas JR, Porreca F (1988) Direct dependence studies in rats with agents selective for different types of opioid receptors. J Pharmacol Exp Ther 246:940–955
Cuomo V, Cagliano R, Desalvia MA, Restani P, Galimberti R, Racagni G, Galli CL (1988) Ultrasonic vocalization in rat pups as a marker of behavioral development: an investigation of the effects of drugs influencing brain opioid systems. Neurotoxicol Teratol 10:465–469
D'Amour F, Smith D (1941) A method for determining loss of pain sensation. J Pharmacol Exp Ther 72:74–79
Dauge V, Rossignol P, Roques BP (1988) Comparison of the behavioral effects induced by administration in rat nucleus accumbens or nucleus caudatus of selectiveμ andδ opioid peptides or kelatorphan an inhibitor of enkephalin-degrading enzymes. Psychopharmacology 96:343–352
Dauge V, Rossignol P, Roques BP (1989) Blockade of dopamine receptors reverses the behavioral effects of endogenous enkephalins in the nucleus caudatus but not in the nucleus accumbens: differential involvement ofδ andμ opioid receptors. Psychopharmacology 99:168–175
Dewey WL, Harris LS (1975) The tail-flick test. In: Neidle A, Ehrenpreis S (eds), Methods in narcotics research. Dekker, New York, (pp 101–109)
Fowler CJ, Fraser GL (1994)μ-,δ-,δ-Opioid receptors and their subtypes. A critical review with emphasis on radioligand binding experiments. Neurochem Int 24:401–426
Galligan JJ, Mosberg HI, Hurst R, Hruby VJ, Burks TF (1984) Cerebral delta opioid receptors mediate analgesia but not the intestinal motility effects of intracerebroventricularly administered opioids. J Pharmacol Exp Ther 229:641–648
Hammond DL (1989) Inference of pain and its modulation from simple behaviors. In: Charpman CR, Loeser JD (eds) Issues in pain research and therapy: Issues in pain measurement (vol. 12). Raven Press, New York, pp 69–91
Handler CM, Geller EB, Adler MB (1992) Effect of mu-, kappa-and delta-selective opioid agonists on thermoregulation in the rat. Pharmacol, Biochem Behav 43:1209–1216
Haney M, Miczek KA (1993) Ultrasounds during agonistic interactions between female rats (Rattus norvegicus). J Comp Psychol 107:373–379
Haney M, Miczek KA (1994) Ultrasounds emitted by female rats during agonistic interactions: effects of morphine and naltrexone. Psychopharmacology 114:441–448
Heyman JS, Jiang Q, Rothman RB, Mosberg HI, Porreca F (1989) Modulation ofμ-mediated antinociception byδ agonists: characterization with antagonists. Eur J Pharmacol 169:43–52
Hofer MA, Shair H (1978) Ultrasonic vocalization during social interaction and isolation in 2-week-old rats. Dev Psychobiol 11:495–504
Jackson HC, Ripley TL, Nutt DJ (1989) Exploringδ-receptor function using the selective opioid antagonist naltrindole. Neuropharmacology 28:1427–1430
Jurgens U, Pratt R (1979) Role of the periaqueductal gray in vocal expression of emotion. Brain Res 167:367–378
Kafka MS, Wirz-Justice A, Naber D, Moore RY, Benedito MA (1983) Circadian rhythms in rat brain neurotransmitter receptors. Fed Proc 42:2796–2801
Kaltwasser MT (1990) Acoustic signaling in the black rat (Rattus rattus). J Comp Psychol 104:227–232
Kovacs GL, Nyolczas N, Krivan M, Gulya K (1988) Analgesic and tolerance-inducing effects of the highly selective delta opioid agonist [d-Pen2,d-Pen5] enkephalin in mice. Eur J Pharmacol 150:347–353
Lore R, Flannelly K, Farina P (1976) Ultrasounds produced by rats accompany decreases in intraspecific fighting. Aggress Behav 2:175–181
Mansour A, Khachaturian H, Lewis ME, Akil H, Watson HJ (1988) Anatomy of CNS opioid receptors. Trends Neurosci 7:308–314
Miczek KA (1982) Ethological analysis of drug action on aggression, defense and defeat. In: Spiegelstein MY, Levy A. (eds) Behavioral models and analysis of drug action. Elsevier, Amsterdam, pp 225–239
Miczek KA, Tornatzky W, Vivian J (1991) Ethology and neuropharmacology: Rodent Ultrasounds. In: Olivier B, Mos J, Slanger JL (eds) Animal Models in Psychopharmacology, Birkhäuser, Basel, pp 409–429
Miczek KA, Weerts EM, Tornatzky W, DeBold JF, Vatne TM (1992) Alcohol and “bursts” of aggressive behavior: ethological analysis of individual differences in rats. Psychopharmacology 107:551–563
Miczek KA, Weerts EM, Vivian JA, Barros H (1995) Aggression, anxiety and vocalizations in animals: GABAA and 5-HT anxiolytics. Psychopharmacology (in press)
Myers RD (1971) Methods for chemical stimulation of the brain. In: Meyers RD (ed) Methods in Psychobiology, V. 1. Academic press, New York, pp 247–280
Narita M, Suzuki T, Funada M, Misawa M, Nagase H (1993) Involvement ofδ-opioid receptors in the effects of morphine on locomotor activity and the mesolimbic dopaminergic system in mice. Psychopharmacology 111:423–426
Portoghese PS, Sultana M, Takemori AE (1988) Naltrindole, a highly selective and potent non-peptideδ opioid receptor antagonist. Eur J Pharmacol 146:185–186
Sales GD (1972) Ultrasound and aggressive behavior in rats and other small mammals. Anim Behav 20:88–100
Sales GD (1974) Ultrasound in rodents. In: Sales GD, Pye JD (eds), Ultrasonic communication by animals. London, Chapman & Hall, pp 149–200
Scherer KR (1986) Vocal affect expression: a review and a model for future research. Psychol Bull 99:143–165
Schmauss C (1987) Spinal k-opioid receptor-mediated antinociception is stimulus-specific. Eur J Pharmacol 137:197–205
Schmauss C, Yaksh TL (1984) In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther 228:1–12
Sewell GD (1967) Ultrasound in adult rodents. Nature 215:512
Shaikh MB, Lu C-L, Siegel A (1991) Affective defense behavior elicited from the feline midbrain periaqueductal gray is regulated byμ andδ opioid receptors. Brain Res 557:344–348
Sharif NA, Hughes J (1989) Discrete mapping of brain mu and delta opioid receptors using selective peptides: quantitative autoradiography, species differences and comparison with kappa receptors. Peptides 10:499–522
Shepherd JK, Blanchard DC, Weiss SM, Rodgers RJ, Blanchard RJ (1992) Morphine attenuates antipredator ultrasonic vocalizations in mixed-sex rat colonies. Pharmacol Biochem Behav 41:551–558
Siegel A, Pott CB (1988) Neural substrates of aggression and flight in the cat. Prog Neurobiol 31:261–283
Suh HH, Tseng LF (1990) Lack of antinociceptive cross-tolerance between intracerebroventricularly administeredβ-endorphin and morphine or DPDPE in mice. Life Sci 46:759–765
Takahashi LK, Thomas DA, Barfield R (1983) Analysis of ultrasonic vocalizations emitted by residents during aggressive encounters among rats (Rattus norvegicus). J Comp Psychol 97:207–212
Thomas DA, Takahashi LK, Barfield RJ (1983) Analysis of ultrasonic vocalization emitted by intruders during aggressive encounters among rats (Rattus norvegicus). J Comp Psychol 97:201–206
Tonoue T, Ashida Y, Makino H, Hata H (1986) Inhibition of shockelicited ultrasonic vocalization by opioid peptides in the rat. A psychotropic effect. Psychoneuroendocrinology 11:177–184
Tornatzky W, Miczek KA (1995) Alcohol, anxiolytics and social stress in rats. Psychopharmacology (in press)
Van der Poel AM, Miczek KA (1991) Long ultrasonic calls in male rats following mating, defeat and aversive stimulation: frequency modulation and bout structure. Behaviour 119:127–142
Vezina P, Kalivas PW, Stewart J (1987) Sensitization occurs to the locomotor effects of morphine and the specificμ opioid receptor agonist, DAGO, administered repeatedly to the ventral tegmental area but not to the nucleus accumbens. Brain Res 417:51–58
Vivian JA, Miczek KA (1993a) Morphine attenuates ultrasonic vocalizations during agonistic encounters in adult rats. Psychopharmacology 111:66–73
Vivian JA, Miczek KA (1993b) Diazepam and gepirone selectively attenuate either 20–32 or 32–65 kHz ultrasonic vocalizations during aggressive encounters. Psychopharmacology 112:66–73
Wetzel DM, Kelley DB, Campbell BA (1980) Central control of ultrasonic vocalizations in neonatal rats: I. Brain stem motor nuclei. J Comp Physiol Psychol 94:596–605
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Haney, M., Miczek, K.A. Delta opioid receptors: reflexive, defensive and vocal effective responses in female rats. Psychopharmacology 121, 204–212 (1995). https://doi.org/10.1007/BF02245631
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02245631