Abstract
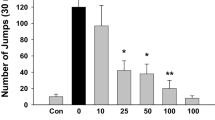
The role of sodium cromoglycate (CRO) on analgesia, locomotor activity and morphine withdrawal in mice was studied in morphine-dependent and drugnaive mice. CRO (0.5, 1, 5, 10, 30, 50 and 100 mg/kg, SC) induces analgesia (hot plate), an effect blocked by previous administration of the opiate antagonist naloxone (1 mg/kg). Furthermore, CRO (30 mg/kg) potentiates morphine analgesia. In morphine-tolerant mice, moderate doses of CRO (0.5, 1, 5, 10 and 30 mg/kg) do not induce analgesia, which suggested the development of cross tolerance between CRO and morphine, whereas coadministration of CRO and morphine in morphine tolerant animals restored the sensitivity to morphine. Administration of CRO (10 and 30 mg/kg) induces an increase in spontaneous locomotor activity, and previous administration of naloxone (1 mg/kg) blocks this effect, whereas CRO (10 mg/kg) blocks morphine (10 mg/kg) and amphetamine (3 mg/kg)-induced hyperactivity. CRO (10, 50 and 100 mg/kg) induces a significant and dose-dependent reduction in the number of jumps (“jumping up”) during naloxone (1 mg/kg)-induced withdrawal in morphine-dependent mice. Finally, CRO (100 mg/kg) reduces the “wet dog shake” phenomenom during naloxone-induced withdrawal in morphine-dependent mice. These results suggest a possible stabilizing effect of CRO on the membranes of neurones that mediate analgesia, locomotor activity and opiate abstinence. Changes and inhibition of DA, NA and 5-HT release may also explain these effects.
Similar content being viewed by others
References
Aufrere MB, Anderson LD (1980) Analgesic effect of continuous intracerebral infusion of guanosine 3′–5′-monophosphate in the rat. Fed Proc Fed Am Soc Exp Biol 39:760
Bensemana D, Gascon AL (1978) Relationship between analgesia and turnover of brain biogenic amines. Can J Physiol Pharmacol 56:721–730
Bhargava HN (1981) Mechanism of toxicity and rationale for use of the combination of pentazocine and pyribenzamine in morphine-dependent subjects. Clin Toxicol 18:175–188
Bhargava HN, Way EL (1972) Acethylcholinesterase inhibition and morphine effects in morphine tolerant and dependent mice. J Pharmacol Exp Ther 183:31–40
Bongianni F, Carla V, Moroni F, Pellegrini-Giampietro DE (1986) Calcium-channel inhibitors supress the morphine-withdrawal syndrome in rats. Br J Pharmacol 88:561–567
Botting R, Morinan A (1982) Involvement of 5-hydroxytryptamine in the analgesic action of pethidine and morphine in mice. Br J Pharmacol 75:579–585
Carroll BJ, Sharp PT (1972) Monoamine mediation of morphine-induced activation of mice. Br J Pharmacol 46:124–139
Church MK (1978) Cromoglycate-like anti-allergic drugs: a review. Drugs of Today 14:281–341
Diaugustine RP, Lazarus LH, Jahnke G, Khan MN, Erisman MD, Linnoila RI (1980) Corticotropin/beta-endorphin immunoreactivity in rat mast cells. Peptide or protease?. Life Sci 27:2663–2668
Foreman JC, Mongar JL, Gomperts BD, Garland LG (1974) A possible role for cyclic AMP in the regulation of histamine secretion and the action of cromoglycate. Biochem Pharmacol 24:538–540
Gibson RD, Tingstad TE (1970) Formulation of a morphine implantation pellet suitable for tolerance-physical dependence studies in mice. J Pharm Sci 59:426–427
Grzanna R, Mollevier ME (1980) The locus coeruleus in the rat: an immunuhistochemical delineation. Neuroscience 5:21–40
Grzanna R, Shultz LD (1982) The contribution of mast cells to the histamine content of the central nervous system: a regional analysis. Life Sci 30:1959–1964
Herz A, Bläsig J, Papeschi R (1974) Role of catecholaminergic mechanisms in the expression of the morphine abstinence syndrome in rats. Psychopharmacology 39:121–143
Hui FW, Sun CLJ, Tocus EC, Hanig JP (1983) The effect of tripelennamine alone and in combination with opiates to produce antinociception in mice. Life Sci 32:1531–1538
Leza JC, Lizasoain I, Martin MI, Lorenzo P (1988) Effects of nifedipine, verapamil and diltiazem on naloxone-induced abstinence in morphine-dependent mice. Rev Farmacol Clin Exp 5:383–387
Leza JC, Lizasoain I, Lorenzo P (1990a) Effects of antihistaminics on naloxone-induced withdrawal in morphine-dependent mice. Psychopharmacology 102:106–111
Leza JC, Lizasoain I, Lorenzo P (1990b) H1- and H2-histamine receptors blockers and opiate analgesia in mice. Methods Find Exp Clin Pharmacol 12:671–678
Leza JC, Lizasoain I, Lorenzo P (1991) Effects of antihistaminics on locomotor activity in mice. Comparison with opiate and amphetamine-induced hyperactivity. Gen Pharmacol 22:293–296
Licata SP, Nalwalk JW, Hough LB (1990) Differential effects of morphine on histamine metabolism in brain and spinal cord of mice. Brain Res 521:125–130
Lidbrink P, Johnsson G, Fuxe K (1971) The effect of imipramine-like drugs and antihistamine drugs on uptake mechanisms in the central noradrenaline and 5-hydroxytryptamine neurons. Neuropharmacology 10:521–536
Malec D, Langwinski R (1983) The effects of antihistaminics on cataleptogenic action of analgesics and haloperidol. Pol J Pharmacol Pharm 35:293–300
Martin MI, Lizasoain I, Leza JC (1990) Calcium channel blockers: effects on morphine-induced hypermotility. Psychopharmacology 101:267–270
Maruyama J, Takemori AE (1973) The role of dopamine and noreprinephrine in the naloxone-induced abstinence of morphine-dependent mice. J Pharmacol Exp Ther 185:602–608
McNeill JH (1980) Histamine receptors and cyclic AMP. Can J Physiol Pharmacol 58:1023–1030
Mickley GA (1986) Histamine H2 receptors mediate morphine-induced locomotor hyperactivity of the C57B1/6j mouse. Behav Neurosci 100:79–84
Navarro M, Lizasoain I, Leza JC, Lorenzo P (1991) An original method for assessment of the jumping test. Methods Find Exp Clin Pharmacol 13:443–447
Pearce FL, Al-Laith M, Bosman L, Brostoff J, Cunniffe TM, Flint KC, Hudspith BN, Jaffar ZH, Johnson NM, Kassessinoff TA, Lau HYA, Lee PY, Leung KBP, Liu WL, Tainnsh KR (1989) Effects of sodium cromoglycte and nedocromil sodium on histamine secretion from mast cells from various locations. Drugs 37:37–43
Picatoste F, Blanco I, Palacios JM (1977) The presence of two cellular pools of rat brain histamine. J Neurochem 29:735–737
Poklis A (1978) T's and blues. JAMA 240:108
Poling A, Sewell RG, Gallus JA, Nearchov NI (1985) Lethality of opioid and antihistamine combinations in mice. Pharmacol Biochem Behav 22:333–335
Pranzatelli MR (1989) Benzodiazepine-induced shaking behavior in the rat: structure-activity and relation to serotonin and benzodiazepine receptors. Exp Neurol 104:241–250
Romandini S, Cervo L, Samanin R (1984) Evidence that drugs increasing 5-hydroxytryptamine transmission block jumping but not wet dog shakes in morphine-abstinent rats: a comparison with clonidine. J Pharm Pharmacol 36:68–70
Saelens JK, Granat FR, Sawyer WK (1971) The mouse jumping test. A simple screening method to estimate the physical dependence capacity of analgesics. Arch Int Pharmacodyn Ther 190:213–218
Sansone M, Castellano C, Libri V (1988) Tripelennamine enhances buprenorphine-, but not pentazocine-induced hyperactivity in mice. Psychopharmacology 85:176–179
Showalter CV (1980) T's and blues. JAMA 244:1224–1225
Strang J (1985) Abuse of buprenorphine. Lancet II:275
Sun CLJ, Hiu FW, Hänig JP (1985) Effects of the H1 blockers alone and in combination with morphine to produce antinociception in mice. Neuropharmacology 24:1–4
Tallarida RJ, Murray RB (1981) Manual of pharmacologic calculations with computer programs. Springer, Berlin Heidelberg New York, pp 54–55
Theoharides TC (1990) Mast cells: the immune gate to the brain. Life Sci 46:607–617
Turski W, Czuczwar SJ, Turski L, Kleinrok Z (1982) The involvement of catecholaminergic mechanisms in the appearance of wet dog shakes produced by carbachol chlorhide in rats. Arch Int Pharmacodyn Ther 225:204–211
Ukai M, Kameyama T (1985) Naloxone specifically blocks the linear locomotion in mice. Brain Res 328:378–380
Waller DP, Katz NL, Morris RW (1980) Potentiation of lethality in mice by combinations of pentazocine and tripelennamine. Clin Toxicol 16:17–23
Wells E, Mann J (1983) Phosphorylation of a mast cell protein in response to treatment with anti-allergic compounds. Implications for the mode of action of sodium cromoglycate. Biochem Pharmacol 32:837–842
Winslow JT, Miczek KA (1988) Naltrexone blocks amphetamine-induced hyperactivity, but not disruption of social and agonistic behavior in mice and squirrel monkeys. Psychopharmacology 96:493–499
Yeh SI (1983) The effect of tripelennamine and other antihistaminic drugs on pentazocine analgesia in the rat. Fed Proc 42:1017
Author information
Authors and Affiliations
Additional information
This work was supported by a grant from the CICYT (Program FAR 90/0543).
Rights and permissions
About this article
Cite this article
Leza, JC., Lizasoain, I., San Martín-Clark, O. et al. Role of sodium cromoglycate on analgesia, locomotor activity and opiate withdrawal in mice. Psychopharmacology 107, 595–600 (1992). https://doi.org/10.1007/BF02245276
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02245276