Abstract
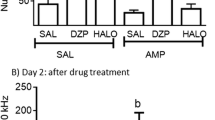
Rats were pretreated for 2 weeks with similarly effective doses of the typical neuroleptic fluphenazine (FPZ) or the experimental weak partial D2 agonists S(+)N-n-propylnorapomorphine (NPA) and S(+)11-hydroxy-N-n-propylnoraporphine (11-OH-NPa). Spontaneous and dopamine (DA) agonist (apomorphine; APO) stimulated stereotyped behaviors or locomotion, and interactions with APO were evaluated over the following 2 weeks. While FPZ induced marked supersensitivity in APO stereotypy, (+)NPA showed no significant change, and (+)11-OH-NPa produced only a small, transient increase in response; NPA also lacked a supersensitizing effect on locomotor arousal induced by APO. The time-course of stereotyped responses to APO following pretreatment with FLZ included a marked increase following FPZ that became maximal at day 5 and normalized by day 9; there was a parallel reduction of acute antistereotypy efficacy of FPZ. (+)11-OH-NPa had similar, but much lesser and shorter-lived effects. Spontaneous locomotion was markedly depressed following FPZ, recovered in 1 week, exceeded controls at day 9, and returned to baseline by day 11; (+)11-OH-NPa, again, had similar but smaller effects. Acute effects of FPZ to reduce spontaneous or APO-induced locomotion were greater after FPZ pretreatment and normalized within a week; (+)11-OH-NPa had a similar but smaller effect. Locomotor arousal by APO was altered inconsistently in the week after pretreatment with FPZ or (+)11-OH-NPa. Thus, FPZ appeared to induce tolerance and supersensitivity in central DA systems, most clearly seen following a several-day period to eliminate the drug. In contrast, the S(+)aporphines had negligible, or minor and transient, effects of a similar kind. These findings support proposals of S(+)aporphines or other D2 partial agonists as potential atypical antipsychotic agents with low risk of inducing long-term adaptive changes in DA receptor sensitivity associated with typical neuroleptic agents.
Similar content being viewed by others
References
Arana GW, Goff D, Baldessarini RJ (1988) Efficacy of anticholinergic prophylaxis of neuroleptic-induced acute dystonia. Am J Psychiatry 145:993–996
Asper H, Baggiolini M, Burki HR, Lauener H, Ruch W, Stille G (1973) Tolerance phenomena with neuroleptics: catalepsy, apomorphine stereotypies and striatal dopamine metabolism in the rat after single and repeated administration of loxapine and haloperidol. Eur J Pharmacol 22:287–294
Bacapoulos NG, Bustos G, Redmond DE, Baulu J, Roth RH (1978) Regional sensitivity of primate brain dopaminergic neurons to haloperidol: alterations following chronic treatment. Brain Res 157:369–401
Baldessarini RJ (1990) Drugs and the treatment of psychiatric disorders. In: Gilman AG, Goodman LS, Rall TW, Murad F (eds) Goodman and Gilman's The pharmacologic basis of therapeutics. Pergamon Press, New York, pp 383–435
Baldessarini RJ, Cohen BM, Teicher MH (1990) Pharmacological treatment. In: Levy ST, Ninan PT (eds) Schizophrenia: treatment of acute psychotic episodes. American Psychiatric Press, Washington, DC, pp 61–118
Baldessarini RJ, Cole JO, Davis JM, Gardos G, Simpson G, Tarsy D (1980) Tardive dyskinesia. American Psychiatric Association, Washington, DC
Baldessarini RJ, Frankenburg F (1991) Clozapine — a novel antipsychotic drug. N Engl J Med 324:746–754
Baldessarini RJ, Tarsy D (1979) Relationship of the actions of neuroleptic drugs to the pathophysiology of tardive dyskinesia. Int Rev Neurobiol 21:1–45
Bannon MJ, Reinhard JF Jr, Bunney EB, Roth RH (1982) Mesocortical dopamine neurons: unique response to antipsychotic drugs explained by absence of terminal autoreceptors. Nature 296:444–446
Booth RG, Baldessarini RJ, Kula NS, Gao Y, Zong R, Neumeyer JL (1990) Presynaptic inhibition of dopamine synthesis in rat striatal tissue by enantiomeric mono- and dihydroxyaporphines. Mol Pharmacol 38:92–101
Booth RG, Baldessarini RJ, Campbell A (1991) Inhibition of dopamine synthesis in rat striatal minces: evidence of dopamine autoreceptor supersensitivity to S(+) but not R(−) N-n-propylnorapomorphine after pretreatment with fluphenazine. Biochem Pharmacol 41:2040–2043
Campbell A, Baldessarini RJ (1981) Tolerance of behavioral effects of prolonged administration of haloperidol. Life Sci 29:1341–1346
Campbell A, Baldessarini RJ, Cremens C, Teicher MH, Marsh E (1989) Interactions between bromocriptine and cocaine: prevention of behavioral arousal and depression in the rat. Neuropsychopharmacology 2:209–224
Campbell A, Baldessarini RJ, Gao Y, Ram VJ, Neumeyer JL (1990) R(−) and S(+) stereoisomers of 11-hydroxy-N-n-propylnoraporphine: central dopaminergic behavioral activity in the rat. Neuropharmacology 29:527–536
Campbell A, Baldessarini RJ, Kula NS (1985a) Prolonged antidopamine actions of single doses of butyrophenones in the rat. Psychopharmacology 87:161–166
Campbell A, Baldessarini RJ, Kula NS, Ram VJ, Neumeyer JL (1987) S(+)methylenedioxy-N-n-propylnoraporphine: an orally active inhibitor of dopamine selective for rat limbic system. Brain Res 403:393–397
Campbell A, Baldessarini RJ, Stoll A, Teicher MH, Maynard P (1984) Effect of age on behavioral responses and tissue levels of apomorphine in rat. Neuropharmacology 23:725–730
Campbell A, Baldessarini RJ, Teicher MH, Neumeyer JL (1985b) S(+)aporphines: selective inhibition of excitatory effects of dopamine injected into rat limbic system. Neuropharmacology 24:391–399
Campbell A, Baldessarini RJ, Teicher MH, Neumeyer JL (1986) Behavioral effects of apomorphine isomers in the rat: selective locomotor-inhibitory effects of S(+)N-n-propylnorapomorphine. Psychopharmacology 88:158–164
Campbell A, Baldessarini RJ, Yeghiayan S (1992) Antagonism of dopamine in regions of rat brain by ergolines. Brain Res 592:348–352
Campbell A, Yeghiayan S, Baldessarini RJ, Neumeyer JL (1991) Selective antidopaminergic effects of S(+)N-n-propylnoraporphines in limbic vs. extrapyramidal sites in rat brain: comparisons with typical and atypical antipsychotic agents. Psychopharmacology 103:323–329
Chouinard G, Jones BB, Annabale L (1978) Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry 135:1409–1410
Clark D, White FJ (1987) A critical evaluation of the D1/D2 dopamine receptor classification and its functional implications. Synapse 1:347–388
Cohen BM, Baldessarini RJ, Campbell A, Tsuneizumi T, Babb S (1992) Persistence of antipsychotic drug effects and tissue levels. Psychopharmacology 108:338–344
Costall B, Naylor RJ, Cannon JG, Lee T (1977) Differentiation of the dopamine mechanism mediating stereotyped behavior and hyperactivity in the nucleus accumbens and caudate-putamen. J Pharm Pharmacol 29:337–342
Coward DM, Dixon AK, Urwyler S, White TG, Enz A, Karobath M, Sherman G (1990) Partial dopamine-agonistic and atypical neuroleptic properties of the amino-ergolines SDZ-208–911 and SDZ-208–912. J Pharmacol Exp Ther 252:279–285
Cox RF, Neumeyer JL, Waszcak BL (1988) Effects of N-n-propylnorapomorphine enantiomers on single unit activity of substantia nigra pars compacta and ventral tegmental area dopamine neurons. J Pharmacol Exp Ther 247:355–362
Creese I, Snyder SH (1979) Nigrostriatal lesions enhance striatal3H-apomorphine and3H-spiroperidol binding. Eur J Pharmacol 56:277–281
Figur LM, Evans DL, Stratman NC, Lahti RA (1992) The dopamine D4 and D2 receptors: comparison of neuroleptic binding affinities (No 158.3). Neurosci Soc Abstr 18:375
Gao Y, Zong R, Campbell A, Kula, NS, Baldessarini RJ, Neumeyer JL (1988) Synthesis and dopamine agonist and antagonist effects of R(−)- and S(+)11-hydroxy-N-n-propylnoraporphine. J Med Chem 31:1392–1396
Grace AA (1991) Phasic vs. tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 41:1–24
Hedberg A, Gerber JG, Nies AS, Wolfe BB, Molinoff PB (1986) Effects of pindolol and propranolol on beta adrenergic receptors on human lymphocytes. J Pharmacol Exp Ther 239:117–123
Karobath M, Coward DM, Enz A, Haefliger W, Jaton A, Markstein R, Palacios JM, Sherman G, Supavilai P, Wiederhold KH, Vigouret J-M (1988) Functional effects of partial D2 dopamine receptor agonists. In: Dahlström A, Belmaker R, Sandler M (eds) Progress in catecholamine research, central aspects. Liss, New York, pp 19–26
Klawans HL Jr (1973) The pharmacology of tardive dyskinesia. Am J Psychiatry 130:82–86
Mandel RJ, Wilcox RE, Randall PK (1992) Behavioral quantification of striatal dopaminergic supersensitivity after bilateral 6-hydroxydopamine lesions in the mouse. Pharmacol Biochem Behav 41:343–347
Markstein R, Seiler MP, Jaton A, Briner U (1992) Structure activity relationship and therapeutic uses of dopaminergic ergots. Neurochem Int 20 [Suppl]: 211S-214S
Marsden CD, Tarsy D, Baldessarini RJ (1975) Spontaneous and drug-induced movement disorders in psychotic patients. In: Benson DF, Blumer D (eds) Psychiatric aspects of neurological disease. Grune & Stratton, New York, pp 219–265
Mackensie RG, Zigmond MJ (1985) Chronic neuroleptic treatment increases D-2 but not D-1 receptors in rat striatum. Eur J Pharmacol 113:159–163
Muller P, Seeman P (1978) Dopaminergic supersensitivity after neuroleptics: time-course and specificity. Psychopharmacology 60:1–11
Neumeyer JL, Reischig D, Arana GW, Campbell A, Baldessarini, Kula NS, Watling KG (1983) Enantioselectivity of R(−) and S(+) N-n-propylnorapomorphine on dopamine receptors. J Med Chem 26:516–521
Neumeyer JL, Froimowitz M, Baldessarini RJ, Campbell A, Gao Y (1988) Neuropharmacology and stereochemistry of dopamine receptor agonist and antagonist enantiomeric pairs. J Recept Res 8[1–4]: 83–96
Porceddu ML, Ongini E, Biggio G (1985) [3H]SCH-23390 binding sites increase after chronic blockade of D-1 dopamine receptors. Eur J Pharmacol 118:367–370
Rangno RE, Langlois S (1982) Comparison of withdrawal phenomena after propranolol, metoprolol, and pindolol. Am Heart J 104:473–478
Riffee WH, Wilcox RE, Smith RV, Davis PJ, Brubaker A (1982) Inhibition of R(−)apomorphine-induced stereotypic cage-climbing behavior in mice by S(+)apomorphine. Adv Biosci 37:357–362
Roth RH, Bacapoulos NG, Bustos G, Redmond DE (1980) Antipsychotic drugs: differential effects on dopamine neurons in basal ganglia and neocortex following chronic administration in human and nonhuman primates. Adv Biochem Psychopharmacol 24:513–520
Seeman P (1980) Brain dopamine receptors. Pharmacol Rev 32:229–313
Shelkunov EL (1967) Adrenergic effect of chronic administration of neuroleptics. Nature 214:1210–1212
Staunton DA, Wolfe BB, Groves PM, Molinoff PB (1981) Dopamine receptor changes following destruction of the nigrostriatal pathway: lack of a relationship to rotational behavior. Brain Res 211:315–327
Staunton DA, Magistretti PJ, Koob GF, Shoemaker WJ, Bloom FE (1982) Dopaminergic supersensitivity induced by denervation and chronic receptor blockade is additive. Nature 299:72–74
Tarsy D, Baldessarini RJ (1973) Pharmacologically induced behavioral supersensitivity to apomorphine. Nature 245:262–263
Tarsy D, Baldessarini RJ (1974) Behavioral supersensitivity to apomorphine following chronic treatment with drugs which interfere with the synaptic function of catecholamines. Neuropharmacology 13:927–940
Wheeler SC, Roth RH (1980) Tolerance to fluphenazine and supersensitivity to apomorphine in central dopaminergic systems after chronic fluphenazine decanoate treatment. Naunyn-Schmiedeberg's Arch Pharmacol 317:151–159
Wolf ME, Roth RH (1987) Dopamine autoreceptors. In: Creese I, Fraser CM (eds) Dopamine receptors. Liss, New York, pp 45–96
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Campbell, A., Baldessarini, R.J. & Neumeyer, J.L. Altered spontaneous behavior and sensitivity to apomorphine in rats following pretreatment with S(+)-aporphines or fluphenazine. Psychopharmacology 111, 351–358 (1993). https://doi.org/10.1007/BF02244952
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02244952