Abstract
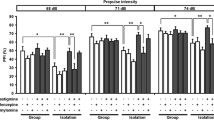
The effect of acetylcholinesterase inhibitors on free swim behavior in rats pretreated with scopolamine (0.32 mg/kg, IP) was examined. Long-Evans rats received a single 5-min testing trial in a 1.5 m black swimming pool, and swim distance in three concentric annulus corridors (peripheral, middle, and inner) and the number of body-turn transitions (>45°) were measured. Physostigmine (1.0 mg/kg, IP) increased swim distance in the middle and inner annulus corridors, compared to tetrahydroaminoacridine (2.0 mg/kg and 10 mg/kg, IP) and scopolamine alone (control) (Ps<0.01), and increased body-turn transitions, compared to all the other groups (Ps<0.05), but had no significant effect on peripheral annulus corridor swim distance, total swim distance, or swim speed. The results suggest that physostigmine produces uniquely different free swim patterns from tetrahydroaminoacridine following cholinergic blockade. These findings have implications for investigations attempting to restore spatial learning and navigation (e.g., Morris water maze) using acetylcholinesterase inhibitors following experimentally-induced cholinergic losses.
Similar content being viewed by others
References
Beatty WW, Bierley RA (1985) Scopolamine degrades spatial working memory but spares spatial reference memory: dissimilarity of anticholinergic effect and restriction of distal visual cues. Pharmacol Biochem Behav 23:1–6
Buresova O, Bolhuis JJ, Bures J (1986) Differential effects of cholinergic blockade on performance of rats in the water tank navigation task and in a radial water maze. Behav Neurosci 100:476–482
Collerton D (1986) Cholinergic function and intellectual decline in Alzheimer's disease. Neuroscience 19:1–28
Davis RE, Coughenour LL, Gamzu E, Moos WH, Schwarz RD (1987) Evaluation of the central pharmacology of tacrine (tetrahydroaminoacridine: THA). Soc Neurosci Abstr 13:487
Dokla CPJ, Parker SC, Thal LJ (1989) Tetrahydroaminoacridine facilitates passive avoidance learning in rats with nucleus basalis magnocellularis lesions. Neuropharmacology 28:1279–1282
Flood JF, Cherkin A (1986) Scopolamine effects on memory retention in mice: a model of dementia? Behav Neural Biol 45:169–184
Fonnum F (1969) Radiochemical micro assays for the determination of choline acetyltransferase and acetylcholinesterase activities. Biochem J 115:465–472
Hallak M, Giacobini E (1989) Physostigmine, tacrine and metrifonate: the effect of multiple doses on acetylcholine metabolism in rat brain. Neuropharmacology 28:199–206
Hunter AJ, Roberts FF (1988) The effect of pirenzepine on spatial learning in the Morris water maze. Pharmacol Biochem Behav 30:519–523
Mandel RJ, Thal LJ (1988) Physostigmine improves water maze performance following nucleus basalis magnocellularis lesions in rats. Psychopharmacology 96:421–425
Patocka J, Bajgar J, Bielavsky J, Fusek J (1976) Kinetics of inhibition of cholinesterases by 1,2,3,4-tetrahydro-9-aminoacridine in vitro. Collection Czechoslov Chem Commun 41:816–824
Schauf CL, Sattin A (1987) Tetrahydroaminoacridine blocks potassium channels and inhibits sodium inactivation inMyxicola. J Pharmacol Exp Ther 243:609–613
Sherman KA, Messamore E (1988) Blood cholinesterase inhibition as a guide to the efficacy of putative therapies for Alzheimer's dementia: comparison of tacrine and physostigmine In: Giacobini E, Becker R (eds) Current research in Alzheimer therapy: cholinesterase inhibitors Taylor & Francis, New York, pp 73–86
Smith G (1988) Animal models of Alzheimer's disease: experimental cholinergic denervation. Brain Res Rev 13:103–118
Stevens R (1981) Scopolamine impairs spatial maze performance in rats. Physiol Behav 27:385–386
Summers WK, Majovski LV, Marsh GM, Tachiki K, Kling A (1986) Oral tetrahydroaminoacridine in long-term treatment of senile dementia, Alzheimer type. N Engl J Med 315:1241–1245
Sutherland RJ, Whishaw IQ, Regehr JC (1982) Cholinergic receptor blockade impairs spatial localization by use of distal cues in the rat. J Comp Physiol Psychol 96:563–573
Watts J, Stevens R, Robinson C (1981) Effects of scopolamine on radial maze performance in rats. Physiol Behav 26:845–851
Whishaw IQ, Tomie J-A (1987) Cholinergic receptor blockade produces impairments in a sensorimotor subsystem for place navigation in the rat: evidence from sensory, motor, and acquisition tests in a swimming pool. Behav Neurosci 101:603–616
Whishaw IQ, O'Connor WT, Dunnett SB (1985) Disruption of central cholinergic systems in the rat by basal forebrain lesions or atropine: Effects on feeding, sensorimotor behaviour, locomotor activity and spatial navigation. Behav Brain Res 17:103–115
Wirsching BA, Beninger RJ, Jhamandas K, Boegman RJ, El-Defrawy SR (1984) Differential effects of scopolamine on working and reference memory of rats in the radial maze. Pharmacol Biochem Behav 20:659–662
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dokla, C.P.J., Rydelek-Fitzgerald, L. Comparison of tetrahydroaminoacridine and physostigmine on scopolamine-induced free swim behavior in the rat. Psychopharmacology 103, 240–243 (1991). https://doi.org/10.1007/BF02244210
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02244210