Abstract
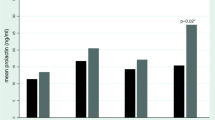
The pharmacokinetics of remoxipride, a new selective dopamine-D2 receptor antagonist with antipsychotic action, was evaluated in eight elderly psychiatric patients with tardive dyskinesia (TD). The daily oral doses of remoxipride were gradually increased from 50 mg per day to 200 mg t.i.d. over 2 weeks. The pharmacokinetics following the initial 50 mg dose (day 1) and the last 200 mg dose (day 15) of the drug were compared in serial samples. Plasma prolactin concentrations were assessed at the same time points. The area under the total plasma concentration versus time curves (AUC) of remoxipride increased proportionally with dose from day 1 to 15. The mean “dose corrected” AUC values for the total concentrations were 96.8 at day 1 (4×24.2, 50 mg single oral dose) and 92.2 µmol·h/l at day 15 (200 mg). The unbound fraction of remoxipride calculated on AUC was slightly higher on day 15 (20%) than on day 1 (15%) (P<0.05), indicating slightly concentration-dependent protein binding of the drug. The mean elimination half-life of total remoxipride was slightly longer on day 15 than day 1 (7.5 versus 5.3 h,P<0.01) The corresponding half-lives for the unbound concentrations were 6.4 and 3.9 h, respectively (P<0.01). The pharmacokinetics of remoxipride is similar in these TD patients and in non-TD patients in previous studies. Following repeated administration of remoxipride, tolerance to the prolactin-releasing action of remoxipride is observed. In plots of the relationship between the concentration of remoxipride and the prolactin increase during a dosage interval, the curve on day 15 was positioned markedly to the right of that on day 1. This indicates a decreased sensitivity to the prolactin releasing action of remoxipride following continuous treatment.
Similar content being viewed by others
References
Andersson U, Nilsson M-I, Häggström J-E, Widerlöv E (1985) Antidyskinetic action and pharmacokinetics of remoxipride, a substituted benzamide, in elderly patients with tardive dyskinesia. In: Burrows G et al. (eds) Clinical and pharmacological studies in psychiatric disorders. Libbey, London, pp 381–388
Andersson U, Häggström J-E, Nilsson M-I, Widerlöv E (1988) Remoxipride, a selective dopamine D2 receptor antagonist, in tardive dyskinesia. Psychopharmacology 94:167–171
Bahr C, von Movin G, Yisak W-A, Jostell K-G, Widman M (1990) Clinical pharmacokinetics of remoxipride. Acta Psychiatr Scand 82 [Suppl 358]
Farde L, Grind M, Nilsson M-I, Ogenstad S, Sedvall G (1988) Remoxipride — a new potential antipsychotic drug. Pharmacological effects and pharmacokinetics following repeated oral administration in male volunteers. Psychopharmacology 95:157–161
Florvall L, Ögren S-O (1982) Potential neuroleptic agent 2,6-dialkoxybenzamide derivatives with potent dopamine receptor blocking activities. J Med Chem 25:1280–1286
Grind M, Nilsson M-I, Nilsson L, Oxenstierna G, Sedvall G, Wahlén A (1989) Remoxipride — a new potential antipsychotic compound. Tolerability and pharmacokinetics after single oral and intravenous administration in healthy male volunteers. Psychopharmacology 98:304–309
Hall H, Sällemark M, Jerning E (1986) Effects of remoxipride and some related new substituted salicylamides on rat brain receptors. Acta Pharmacol Toxicol 58:61–70
Holford NHG, Sheiner LB (1981) Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin Pharmacokinet 6:429–453
Jeste DV, Rosenblatt JE, Wagner RL, Wyatt RJ (1979) High serum neuroleptic levels in tardive dyskinesia? New Engl J Med 301:1184
Lapierre et al. (1990) A controlled dose-ranging study of remoxipride vs. haloperidol in schizophrenia — a Canadian multicentre trial. Acta Psychiatr Scand 82 [Suppl 358]:72–76
Movin G, Gustavsson L, Franzén G, Widerlöv E, Soni SD, Tench D, Nilsson L, Yisak W (1990) Pharmacokinetics of remoxipride in elderly psychotic patients. Acta Psychiatr Scand 82 [Suppl 358]:176–180
Nilsson L (1990) Determination of remoxipride in plasma and urine by reversed-phase liquid chromatography. J Chromatogr 526:139–150
Ögren S-O, Hall H, Köhler C, Magnusson O, Lindblom L-O, Ängeby K, Florvall L (1984) Remoxipride, a new potential antipsychotic compound with selective antidopaminergic actions in the rat brain. Eur J Pharmacol 102:459–474
Schooler NR, Kane JM (1982) Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry 39:486–487
Sigurdsson S (1985) Determination of the free concentration of remoxipride in plasma. Astra Standard Operating Procedure, No. 805-818 AFB-06. (available on request)
Tench D, Soni SD, Ashwood T, Movin G (1990) Steady-state pharmacokinetics of controlled release and immediate release formulations of remoxipride in patients with chronic schizophrenia. Psychopharmacology 101:132–136
Widerlöv E, Häggström J-E, Kilts CD, Andersson U, Breese GR, Mailman RB (1982) Serum concentrations of thioridazine, its major metabolites and serum neuroleptic-like activities in schizophrenics with and without tardive dyskinesia. Acta Psychiatr Scand 66:294–305
Widerlöv E, Termander B, Nilsson M-I (1989) The effect of urinary pH on the plasma and urinary kinetics of remoxipride in man. Eur J Clin Pharmacol 37:359–363
Widerlöv E, Franzén G, Jansson P, Movin G (1990) Pharmacokinetics of remoxipride controlled release and immediate release capsules in schizophrenic patients. Int J Clin Psychopharmacol 5:125–134
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Widerlöv, E., Andersson, U., von Bahr, C. et al. Pharmacokinetics and effects on prolactin of remoxipride in patients with tardive dyskinesia. Psychopharmacology 103, 46–49 (1991). https://doi.org/10.1007/BF02244072
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02244072