Abstract
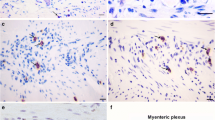
PURPOSE: The mechanisms that control chronic infectionin vivo and the immunologic mechanisms involved in the pathogenesis of chagasic megacolon are not completely characterized. Although autoimmunity may play a role in the pathogenesis of Chagas' disease, recent studies, both in mice and in humans, suggest a positive association of tissue parasitism, inflammation, and severity of lesions. The aim of this study was to evaluate the role of inflammatory cells and the subclasses of lyphocytes involved in neuropathic lesions in the colon of patients who underwent resection for advanced megacolon. METHODS: Specimens from 23 patients were selected based on histopathologic analysis. Paraffin-embedded tissue blocks were sectioned and evaluated by immunohistochemistry for cluster of differentiation 3, cluster of differentiation 8, cluster of differentiation 20, and natural killer cell antibodies by an avidin-biotin peroxidase method. RESULTS: Almost all myenteric plexuses were damaged, characterized by degenerative changes, necrosis of ganglion cells, and inflammatory response. Mild lymphocytic infiltration around degenerated and normal ganglion cells was observed in all cases. Collagen fibers and mononuclear cells surrounded some ganglion cells. Most of the inflammatory cells were lymphocytes, identified as cluster of differentiation 3-positive cells. Cluster of differentiation 8-positive lymphocytes were associated with degenerated ganglion cells. Natural killer cell antibodies were detected in a lower proportion of cells and were distributed between muscle layers or in proximity to the myenteric plexus. All these findings were also observed in the submucosal plexus. Cluster of differentiation 20-positive lymphocytes were not present in muscle layers or in the vicinity of either plexus. CONCLUSION: Pathogenesis of the megacolon is based on a continuous process of ganglion cell damage with participation of T lymphocytes expressing cluster of differentiation 8 and natural killer cell membrane antigens. B lymphocytes do not take part in the chronic inflammatory reaction.
Similar content being viewed by others
References
Chagas C. Ueber eine neue Trypanosomiasis des Menchen. Mem Inst O Cruz 1909;1:159–218.
World Health Organization. Chagas disease. In: Tropical Diseases Research: Progress 1989–1990. Geneva, Switzerland: World Health Organization, 1991:69–77. Tenth Programme Report.
Hudson L, Britten V. Immune response to South American trypanosomiasis and its relationship to Chagas' disease. Br Med Bull 1985;41:175–80.
Olivares-Villagómez D, McCurley TL, Vnencak-Jones CL, Correa-Oliveira R, Colley DG, Carter CE. Polymerase chain reaction amplification of three differentTrypanosoma cruzi DNA sequences from human chagasic cardiac tissue. Am J Trop Med Hyg 1998;59:563–70.
Köberle F. Die Chagaskrankheit, eine Erkrankung der Neurovegetativen Peripherie. Wien Klin Wochenschr 1956;68:333–9.
Martins Campos JV, Tafuri WL. Chagas enteropathy. Gut 1973;14:910–19.
Dias JC. Epidemiologia. In: Brener Z, Andrade Z, Barral-Netto M.Trypanosoma cruzi e Doença de Chagas. 2nd ed. Rio de Janeiro, Brazil: Guanabara & Koogan, 2000.
Tafuri WL, Light and electron microscope studies of the autonomic nervous system in experimental and human American trypanosomiasis. Virchows Arch A Pathol Pathol Anat 1971;354:136–49.
Habr-Gama A, Raia A, Corrêa-Neto A. Motility of the sigmoid colon and rectum: contribution to the physiopathology of megacolon in Chagas' disease. Dis Colon Rectum 1971;14:291–304.
Okumura M, Corrêa-Netto A. Etiopatogenia do megacolo chagásico (contribuição experimental). Rev Hosp Clin Fac Med São Paulo 1963;18:351–60.
Okumura M. Contribuição para o estudo das lesões dos neurônios do plexo mientérico do colo na moléstia de Chagas experimental no camundongo branco. Rev Hosp Clin Fac Med São Paulo 1967;22:192–204.
Tafuri WL. Pathogenesis of lesions of the autonomic nervous system of the mouse in experimental acute Chagas' disease. Am J Trop Med Hyg 1970;19:405–16.
Higuchi MD, Ries MM, Aiello VD, et al. Association of an increase in CD8+ T cells with the presence ofTrypanosoma cruzi antigens in chronic, human, chagasic myocarditis. Am J Trop Med Hyg 1997;56:485–9.
Brodskyn CI, Barral A, Bulhões MA, Souto T, Machado WC, Barral-Netto M. Cytotoxicity in patients with different clinical forms of Chagas' disease. Clin Exp Immunol 1996;105:450–5.
Hsu SM, Raine L, Fanger H. Use of avidin-biotin peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabelled antibody (PAP) procedures. J Histochem Cytochem 1981;29:577–80.
Garcia SB, Paula JS, Giovannetti GS, et al. Nitric oxide is involved in the lesions of the peripheral autonomic neurons observed in the acute phase of experimentalTrypanosoma cruzi infection. Exp Parasitol 1999;93:191–7.
Tarleton RL. Depletion of CD8+ T cells increases susceptibility and reverses vaccine induced immunity in mice infected withTrypanosoma cruzi. J Immunol 1990;144:717–24.
Sunnemark D, Ulfgren AK, Örn A, Harris RA. Cytokine production in hearts ofTrypanosoma cruzi-infected CBA mice: do cytokine patterns in chronic stage reflect the establishment of myocardial pathology? Scand J Immunol 1996;44:421–9.
Cunha-Neto E, Rizzo LV, Albuquerque F, et al. Cytokine production profile of heart-infiltrating T cells in Chagas' disease cardiomyopathy. Braz J Med Biol Res 1998;31:133–7.
Nickell SP, Shyker GA, Arevalo C. Isolation fromT. cruzi infected mice of CD8+ MHC restricted cytotoxic T cells that lyse parasite infected target cells. J Immunol 1993;50:1446–57.
Sato MN, Yamashirokanoshiro EH, Tongi MN, Kaneno R, Higuchi ML, Duarte AG. CD8+ cells and natural cytotoxicity activity among spleen, blood, and heart lymphocytes during the acute phase ofTrypanosoma cruzi infection in rats. Infect Immun 1992;60:1024–30.
Hatcher FM, Kuhn RE, Cerrone MC, Burton RC. Increased natural killer cell activity in experimental American trypanosomiasis. J Immunol 1981;127:1126–31.
Younès-Chennoufi AB, Hontebyrie-Joskowicz M, Tricottet V, Eisen H, Reynes M, Said G. Persistence ofTrypanosma cruzi antigens in the inflammatory lesions of chronically infected mice. Trans R Soc Trop Med Hyg 1988;82:77–83.
Gazzinelli RT, Morato MJ, Nunes RM, Cançado JR, Brener Z, Gazzinelli G. Idiotype stimulation of T lymphocytes fromTrypanosoma cruzi-infected patients. J Immunol 1988;140:3167–72.
Jones EM, Colley DG, Tostes S, Lopes ER, Vnencak-Jones C, McCurley TL. Amplification of aTrypanosoma cruzi DNA sequence from inflammatory lesions in human chagasic cardiomyopathy. Am J Trop Med Hyg 1993;48:348–57.
Russomando G, Figueiredo A, Almirón M, Sakamoto M, Morita K. Polymerase chain reaction-based detection ofTrypanosoma cruzi DNA in serum. J Clin Microbiol 1992;30:2864–8.
Winker P, Britto C, Pereira JB, Cardoso MA, Oelemann W, Morel CM. Use of a simplified polymerase chain reaction procedure to detectTrypanosoma cruzi in blood samples from chronic chagasic patients in a rural endemic area. Am J Trop Med Hyg 1994;51:771–7.
Author information
Authors and Affiliations
Additional information
Supported by FINEP, CNPq, and LIM/5-HCFMUSP. Presented at the Society for Surgery of the Alimentary Tract, Orlando, Florida, May 16 to 19, 1999.
About this article
Cite this article
Corbett, C.E.P., Ribeiro, U., Prianti, M.d.G. et al. Cell-mediated immune response in megacolon from patients with chronic chagas' disease. Dis Colon Rectum 44, 993–998 (2001). https://doi.org/10.1007/BF02235488
Issue Date:
DOI: https://doi.org/10.1007/BF02235488