Abstract
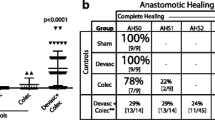
PURPOSE: The mechanisms that cause diabetes to impair the development of anastomotic strength in the intestine are poorly understood. We investigated whether short-term uncontrolled diabetes causes alterations in microscopic aspects of anastomoses from the ileum and colon. METHODS: Eighteen Wistar rats were rendered diabetic one week before operation by intravenous streptozotocin injection (50 mg/kg), resulting in nonfasting blood glucose levels of approximately 20 mmol/l. Another 18 age-matched rats were used as controls with a normal blood glucose range of 5 to 7 mmol/l. All rats underwent resection and anastomosis of both the ileum and colon. Animals were killed at one, three, or seven days after operation. Cellular and architectural parameters of anastomotic healing were scored in hematoxylin and eosin-stained sections. Anastomotic collagen content was analyzed by image analysis in picrosirius red-stained sections. RESULTS: Anastomotic necrosis, edema, and epithelial recovery were not affected by diabetes. In diabetic rats, the number of polymorphonuclear cells and macrophages was significantly (P=0.025 and 0.0002, respectively) increased in ileal anastomoses one and three days after operation. In colonic anastomoses, the number of polymorphonuclear cells was increased at one (P=0.001) and seven (P=0.014) days after operation. Repair of the submucosal-muscular layer in colonic anastomoses from diabetic rats was impaired seven days after surgery (P=0.0071), but in ileal anastomoses no difference was found. In the anastomotic area, collagen deposition at postoperative Days 1, 3, and 7 remained unaffected by diabetes. CONCLUSION: Experimental diabetes leads to alterations in cellular components involved in the early phase of repair of intestinal anastomoses but not to a reduced accumulation of wound collagen.
Similar content being viewed by others
References
Thornton FJ, Barbul A. Healing in the gastrointestinal tract. Surg Clin North Am 1997;77:549–86.
Hunt TK. Comment. In: Hunt TK, ed. Wound healing and wound infection: theory and surgical practice. New York: Appleton-Century-Crofts, 1980:192–3.
Morgenstern L, Yamakawa T, Ben-Shoshan M, Lippman H. Anastomotic leakage after low colonic anastomosis. Clinical and experimental aspects. Am J Surg 1972;123:104–8.
Högström H, Haglund U, Zederfeldt B. Beneficial effect of proteinase inhibitors on early breaking strength of intestinal anastomoses. Acta Chir Scand 1985;151:529–32.
Hendriks T, Mastboom WJ. Healing of experimental intestinal anastomoses: parameters for repair. Dis Colon Rectum 1990;33:891–901.
Paul RG, Tarlton JF, Purslow PP,et al. Biomechanical and biochemical study of a standardized wound healing model. Int J Biochem Cell Biol 1997;29:211–20.
Goodson WH, Hunt TK. Wound healing and the diabetic patient. Surg Gynecol Obstet 1979;149:600–8.
Andreassen TT, Oxlund H. The influence of experimental diabetes and insulin treatments on the biomechanical properties of rat skin incisional wounds. Acta Chir Scand 1987;153:405–9.
Bitar MS, Labbad ZN. Transforming growth factor-β and insulin-like growth factor-I in relation to diabetes-induced impairment of wound healing. J Surg Res 1996;61:113–9.
Goodson WH, Hunt TK. Studies of wound healing in experimental diabetes mellitus. J Surg Res 1977;22:221–7.
Schäffer MR, Tantry U, Efron PA, Ahrendt GM, Thornton FJ, Barbul A. Diabetes-impaired healing and reduced wound nitric oxide synthesis: a possible pathophysiologic correlation. Surgery 1997;121:513–9.
Seifter E, Rettura G, Padawer J, Stratford F, Kambosos D, Levenson SM. Impaired wound healing in streptozotocin diabetes. Prevention by supplemental vitamin A. Ann Surg 1981;194:42–50.
Verhofstad MH, Hendriks T. Diabetes impairs the development of early strength, but not the accumulation of collagen, during intestinal anastomotic healing in the rat. Br J Surg 1994;81:1040–5.
Verhofstad MH, van der Hem LG, van der Vliet JA, Hendriks T. Pancreatic islet transplantation prevents the impaired healing of intestinal anastomoses in streptozotocin-diabetic Lewis rats. Wound Repair Regen 1995;3:221–8.
Verhofstad MH, Hendriks T. Complete prevention of impaired anastomotic healing in diabetic rats requires preoperative blood glucose control. Br J Surg 1996;83:1717–21.
Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 1979;11:447–55.
Rabau MY, Dayan D. Polarization microscopy of picrosirius red stained sections: a useful method for qualitative evaluation of intestinal wall collagen. Histol Histopathol 1994;9:525–8.
Biert J, Seifert WF, Verhofstad AA,et al. A semiquantitative histological analysis of repair of anastomoses in the rat colon after combined preoperative irradiation and local hyperthermia. Radiat Res 1998;149:372–7.
Martens MF, Hendriks T. Postoperative changes in collagen synthesis in rat intestinal anastomoses: differences between small and large bowel. Gut 1991;32:1482–7.
Monnier VM, Bautista O, Kenny D,et al. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensiveversus conventional therapy of type 1 diabetes: relevance of glycated collagen productsversus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes 1999;48:870–80.
Black CT, Hennessey PJ, Ford EG, Andrassy RJ. Protein glycosylation and collagen metabolism in normal and diabetic rats. J Surg Res 1989;47:200–2.
Sannomiya P, Pereira MA, Garcia-Leme J. Inhibition of leukocyte chemotaxis by serum factor in diabetes mellitus: selective depression of cell responses mediated by complement-derived chemoattractants. Agents Actions 1990;30:369–76.
Pereira MA, Sannomiya P, Garcia-Leme J. Inhibition of leukocyte chemotaxis by factor in alloxan-induced diabetic rat plasma. Diabetes 1987;36:1307–13.
Sato N, Shimizu H, Shimomura Y, Uehara Y, Takahashi M, Kobayashi I. Reduced ability of neutrophils to produce active oxygen species in streptozotocin-induced diabetic rats. Exp Clin Endocrinol 1992;99:31–3.
Bagdade JD, Root RK, Bulger RJ. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes 1974;23:9–15.
Wilson RM, Tomlinson DR, Reeves WG. Neutrophil sorbitol production impairs oxidative killing in diabetes. Diabetes Med 1987;4:37–40.
Morohoshi M, Fujisawa K, Uchimura I, Numano F. The effects of glucose and advanced glycosylation end products on IL-6 production by human monocytes. Ann N Y Acad Sci 1995;748:562–70.
Doxey DL, Nares S, Park B, Trieu C, Cutler CW, Iacopino AM. Diabetes-induced impairment of macrophage cytokine release in a rat model: potential role of serum lipids. Life Sci 1998;63:1127–36.
Doxey DL, Ng MC, Dill RE, Iacopino AM. Platelet-derived growth factor levels in wounds of diabetic rats. Life Sci 1995;57:1111–23.
Ries C, Petrides PE. Cytokine regulation of matrix metalloproteinase activity and its regulatory dysfunction in disease. Biol Chem Hoppe Seyler 1995;376:345–55.
Schäffer M, Barbul A. Lymphocyte function in wound healing and following injury. Br J Surg 1998;85:444–60.
Fishel RS, Barbul A, Beschorner WE, Wasserkrug HL, Efron G. Lymphocyte participation in wound healing. Morphologic assessment using monoclonal antibodies. Ann Surg 1987;206:25–9.
Author information
Authors and Affiliations
About this article
Cite this article
Verhofstad, M.H.J., Lange, W.P., van der Laak, J.A.W.M. et al. Microscopic analysis of anastomotic healing in the intestine of normal and diabetic rats. Dis Colon Rectum 44, 423–431 (2001). https://doi.org/10.1007/BF02234744
Issue Date:
DOI: https://doi.org/10.1007/BF02234744