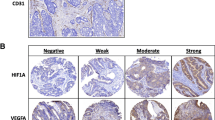
Abstract
PURPOSE: Intratumor microvessel count has been reported as a useful prognostic factor in patients with cancer of various organs. This study was undertaken to clarify the relation between microvessel count and lymph node metastasis in submucosal colorectal cancer. METHODS: Microvessel count was estimated in 254 invasive tumors that had been resected from patients with submucosal colorectal cancer. Immunohistochemistry with antibodies against CD34 was performed on archival specimens, and microvessel counts were estimated based on the average count of three fields (original magnification, ×400) in the most vascular area at the site of deepest submucosal penetration. RESULTS: Microvessel count ranged from 10 to 98, with a median of 40. Lesions with high microvessel counts (≥40) had a significantly higher incidence of lymph node metastasis than those with low microvessel counts (<40; 21.8 percentvs. 6.2 percent). None of the 79 lesions with low microvessel counts and submucosal invasion up to a depth of 1,500µm had metastasized to the lymph nodes. In multivariate analysis, microvessel count was an independent risk factor for lymph node metastasis in submucosal colorectal cancer (P=0.0026). CONCLUSION: Microvessel count at the site of deepest submucosal penetration can be one of the most useful predictors for lymph node metastasis. Analysis that combines microvessel count and depth of submucosal invasion may predict the occurrence of lesions without lymph node metastasis.
Similar content being viewed by others
References
Karita M, Tada M, Okita K, Kodama T. Endoscopic therapy for early colon cancer: the strip biopsy resection technique. Gastrointest Endosc 1991;37:128–32.
Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy 1993;25:455–61.
Yokota T, Sugihara K, Yoshida S. Endoscopic mucosal resection for colorectal neoplastic lesions. Dis Colon Rectum 1994;37:1108–11.
Usui Y, Kudo K, Satou T, Satou M, Hamada S, Kitamura S. A case of type IIc cancer having submucosal venous invasion without direct submucosal invasion. J Jpn Soc Colo-proctol 1991;44:1089–92.
Morson BC, Whiteway JE, Jones EA, Macrae FA, Williams CB. Histopathology and prognosis of malignant colorectal polyps treated by endoscopic polypectomy. Gut 1984;25:437–44.
Kyzer S, Begin LR, Gordon PH, Mitmaker B. The care of patients with colorectal polyps that contain invasive adenocarcinoma. Cancer 1992;70:2044–50.
Minamoto T, Mai M, Ogino T,et al. Early invasive colorectal carcinomas metastatic to the lymph node with attention to their nonpolypoid development. Am J Gastroenterol 1993;88:1035–9.
Tanaka S, Yokota T, Saito D, Okamoto S, Oguro Y, Yoshida S. Clinicopathologic features of early rectal carcinoma and indications for endoscopic treatment. Dis Colon Rectum 1995;38:959–63.
Tanaka S, Haruma K, Teixeira CR,et al. Endoscopic treatment of submucosal invasive colorectal carcinoma with special reference to risk factors for lymph node metastasis. J Gastroenterol 1995;30:710–17.
Tanaka S, Haruma K, Tatsuta S,et al. Proliferating cell nuclear antigen expression correlates with the metastatic potential of submucosal invasive colorectal carcinoma. Oncology 1995;52:134–9.
Aoki R, Tanaka S, Haruma K,et al. MUC-1 expression as a predictor of the curative endoscopic treatment of submucosally invasive colorectal carcinoma. Dis Colon Rectum 1998;41:1262–72.
Teixeira CR, Tanaka S, Haruma K,et al. The clinical significance of the histologic subclassification of colorectal carcinoma. Oncology 1993;50:495–9.
Hiraga Y, Tanaka S, Haruma K,et al. Single carcinoma cells at the deepest invasive portion correlate with metastatic potential of advanced colorectal carcinoma. Int J Oncol 1997;10:1141–5.
Macchiarini P, Fontanini G, Hardin MJ, Squartini F, Angeletti C. Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet 1992;340:145–6.
Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol 1993;143:401–9.
Toi M, Kashitani J, Tominaga T. Tumor angiogenesis is an independent prognostic indicator in primary breast carcinoma. Int J Cancer 1993;57:371–4.
Porschen R, Classen S, Piontek M, Borchard F. Vascularization of carcinomas of the esophagus and its correlation with tumor proliferation. Cancer Res 1994;54:587–91.
Hollingsworth HC, Kohn EC, Steinberg SM, Rothenberg ML, Merino MJ. Tumor angiogenesis in advanced stage ovarian carcinoma. Am J Pathol 1995;147:33–41.
Maeda K, Chung YS, Takatsuka S,et al. Tumor angiogenesis and tumor cell proliferation as prognostic indicators in gastric carcinoma. Br J Cancer 1995;72:319–23.
Tanigawa N, Amaya H, Matsumura M,et al. Extent of tumor vascularization correlates with prognosis and hematogenous metastasis in gastric carcinomas. Cancer Res 1996;56:2671–6.
Tomisaki S, Ohno S, Ichiyoshi Y, Kuwano H, Maehara Y, Sugimachi K. Microvessel quantification and its possible relation with liver metastasis in colorectal cancer. Cancer 1996;77:1722–8.
Tanigawa N, Amaya H, Matsumura M,et al. Tumor angiogenesis and mode of metastasis in patients with colorectal cancer. Cancer Res 1997;57:1043–6.
Nanashima A, Ito M, Sekine I,et al. Significance of angiogenetic factors in liver metastatic tumors originating from colorectal cancers. Dig Dis Sci 1998;43:2634–40.
Teixeira CR, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G. Proliferating cell nuclear antigen expression at the invasive tumor margin predicts malignant potential of colorectal carcinomas. Cancer 1994;73:575–9.
Jass JR, Sobin LH. International Histological Classification of Tumors. 2nd ed. Berlin: Springer-Verlag, 1989:29–33.
Liotta LA, Saidel MG, Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res 1976;36:889–94.
Nagy JA, Brown LF, Senger DR,et al. Pathogenesis of tumor stroma generation: a critical role for leaky blood vessels and fibrin deposition. Biochim Biophys Acta 1989;948:305–26.
Moscatelli D, Gross JL, Rifkin DB. Angiogenetic factors stimulate plasminogen activator and collagenase production by capillary endothelial cells. J Cell Biol 1981; 91:201a. Abstract.
Weidner N, Semple JP, Weich WR, Folkman J. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 1991;324:1–8.
Kuzu I, Bicknell R, Harris AL, Jones M, Gatter KC, Mason DY. Heterogeneity of vascular endothelial cells with relevance to diagnosis of vascular tumors. J Clin Pathol 1992;45:143–8.
Schlingemann RO, Rietveld FJ, de Waal RM,et al. Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Lab Invest 1990;62:690–6.
Parums DV, Cordell JL, Micklem K, Heryet AR, Gatter KC, Mason DY. JC70: a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J Clin Pathol 1990;43:752–7.
Smith SS, Basu PK. Mast cells in corneal immune reaction. Can J Ophthalmol 1970;5:173–83.
Tanaka S, Haruma K, Oh-e H,et al. Possibility of broadening of the indication for endoscopic treatment for submucosally invasive colorectal carcinoma and the risks involved: analysis of cases with submucosally invasive carcinoma treated endoscopically: I to Cho (stomach and intestine) [in Japanese with English abstract] 1999;6:757–64.
Tanaka S, Haruma K, Oh-e H,et al. Conditions of curability after endoscopic resection for colorectal carcinoma with submucosally massive invasion. Oncol Rep 2000;7:783–8.
Tanaka S, Haruma K, Ito M,et al. Detailed colonoscopy for detecting early superficial carcinoma: recent developments. J Gastroenterol 2000;35:121–5.
Author information
Authors and Affiliations
Additional information
Supported in part by the Foundation for Hiroshima Cancer Seminor Grant.
About this article
Cite this article
Oh-e, H., Tanaka, S., Kitadai, Y. et al. Angiogenesis at the site of deepest penetration predicts lymph node metastasis of submucosal colorectal cancer. Dis Colon Rectum 44, 1129–1136 (2001). https://doi.org/10.1007/BF02234633
Issue Date:
DOI: https://doi.org/10.1007/BF02234633