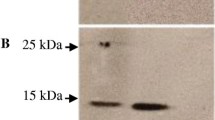
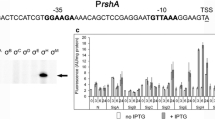
Abstract
In response to the intracellular iron concentrationPseudomonas fluorescens M114 coordinately regulates the production of pseudobactin M114, its cognate receptor PbuA, and a casein protease. Transcriptional initiation of this coordinate iron-stress response requires the sigma factor PbrA. PbrA is a member of the ECF (Extracytoplasmicfunction) subgroup of the σ70 family of eubacterial RNA polymerase sigma factors. Regulatory studies of thepbrA gene utilising promoter-lacZ transcriptional fusions demonstrate that expression ofpbrA dictates the cellular response to iron.pbrA is transcribed in all phases of iron-limited growth but maximally at late-logarithmic to stationary phase.pbrA expression is independent of autoregulatory control but is strictly repressed in iron-rich conditions in a Fur-dependent fashion. Constitutive expression ofpbrA from an inducibletac promoter permits the induction of PbrA-dependent transcription and pseudobactin M114 biosynthesis in high-iron conditions. A PbrA consensus sequence was derived from significant DNA sequence homologies observed within the “ − 25 bp” and “ − 16 bp” regions conserved among all PbrA-dependent promoters. The predicted PbrA target promoter consensus is homologous for the promoter recognition sites for other environmentally responsive ECF sigma factors.
Similar content being viewed by others
References
Adams C, Dowling DN, O'Sullivan DJ, O'Gara F (1994) Isolation of a gene (pbsC) required for siderophore biosynthesis in fluorescentPseudomonas sp. strain M114. Mol Gen Genet 243:515–524
Bagg A, Neilands JB (1987) Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev 51:509–518
Birnboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucl Acids Res 7:1513–1523
Boyer HW, Roulland-Dussoix D (1969) A complementation analysis of restriction and modification inEscherichia coli. J Mol Biol 41:459–472
Cunliffe HE, Merriman TR, Lamont IL (1995) Cloning and characterisation ofpvdS, a gene required for pyoverdine synthesis inPseudomonas aeruginosa: PvdS is probably an alternative sigma factor. J Bacteriol (177) 2744–2750
Deretic V, Schurr MJ, Boucher JC, Martin DW (1994) Conversion ofPseudomonas aeruginosa to mucoidy in cystic fibrosis: Environmental stress and regulation of bacterial virulence by alternative sigma factors. J Bacteriol 176:2773–2780
DeVries CA, Ohman DE (1994) Mucoid-to-nonmucoid conversion in alginate-producingPseudomonas aeruginosa often results from spontaneous mutations inalgT, encoding a putative alternate sigma factor, and shows evidence for autoregulation. J Bacteriol 176:6677–6687
Erickson JW, Gross CA (1989) Identification of the subunit ofEscherichia coli RNA polymerase: a second alternate factor involved in high-temperature gene expression. Genes Dev 3:1462–1471
Figurski DH, Helinski DR (1979) Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA 76:1648–1652.
Gibson TJ (1984) Studies on the Epstein-Barr virus genome. Ph.D. thesis. Cambridge University, Cambridge, UK
Harle C, Kim I, Angerer A, Braun V (1995) Signal transfer through three compartments: transcription initiation of theEscherichia coli ferric citrate transport system from the cell surface. EMBO 14:1430–1438
Helmann JD, Chamberlin MJ (1988) Structure and function of bacterial sigma factors. Annu Rev Biochem 57:839–872
Hutcheson SW, Hen S, Xiao Y (1994) Mechanism for environmental regulation ofPseudomonas syringae pathogenicity and host range determinants. In: Daniels MJ, Downie JA, Osbourn AE (eds) Advances in molecular genetics of plant microbe interactions. vol 3. Kluwer Academic Publishers, Dordrecht, pp 33–36
Koster M, van der Vossenberg J, Leong J, Weisbeek PJ (1993) Identification and characterisation of thepupB gene encoding an inducible ferric-pseudobactin receptor ofPseudomonas putida WCS358, Mol Microbiol 8:591–601
Koster M, van Klompemburg K, Bitter W, Leong J, Weisbeek PJ (1994) Role of the outer membrane ferric siderophore receptor PupB in signal transduction across the bacterial cell envelope. EMBO 13:2805–2813
Lonetto MA, Brown KL, Rudd KE, Buttner MJ (1994) Analysis of theStreptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase σ factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci USA 91:7573–7577
de Lorenzo V, Wee S, Herrero M, Neilands JB (1987) Operator sequences of the aerobactin operon of plasmid Co1V-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol 169:2624–2630
de Lorenzo V, Giovannini F, Herrero M, Neilands JB (1988) Metal ion regulation of gene expression: Fur repressor-operator interaction at the promoter region of the aerobactin system of pCo1V-K30. J Mol Biol 203:875–884
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Martin DW, Holloway BW, Deretic V (1993) Characterisation of a locus determining the mucoid status ofPseudomonas aeruginosa: A1gU shows sequence similarities with aBacillus sigma factor. J Bacteriol 175:1153–1164
Martin DW, Schurr MJ, Yu H, Deretic V (1994) Analysis of promoters controlled by the putative sigma factor A1gU regulating conversion to mucoidy inPseudomonas aeruginosa: relationship to σE and stress response. J Bacteriol 176:6688–6696
Marugg JD, Weisbeek PJ (1991)CRC Handbook of microbial iron chelates. CRC Press, Boca Raton, pp 155–177
McGowan SJ, Gorham HC, Hodgson DA (1993) Light-induced carotenogenesis inMyxococcus xanthus: DNA sequence analysis of thecarR region. Mol Microbiol 10:713–725
Miller JH (1972)Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Morales V, Bagdasarian MM, Bagdasarian M (1989) Promiscuous plasmids of the IncQ group: mode of replication and use for gene cloning in Gram-negative bacteria. In: Silver S, Chakrabarty A, Iglewski B, Kaplan S (eds)Pseudomonas: biotransformations, pathogenesis, and evolving biotechnology. American society for Microbiology, Washington DC, pp 229–241
Morris J, Donnelly D, O'Neill E, McConnell F, O'Gara F (1994) Nucleotide sequence analysis and potential environmental distribution of a ferric pseudobactin receptor gene ofPseudomonas sp. strain M114. Mol Gen Genet 242:9–16
Ochs M, Veitinger T, Kim I, Welz D, Angerer A, Braun V (1995) Regulation of citrate-dependent iron transport ofEscherichia coli: FecR is required for transcription activation by FecI. Mol Microbiol 15:155–165
O'Gara F, Shanmugam KT (1976) Regulation of nitrogen fixation byRhizobia. Export of fixed N2 as NH4 +. Biochem Biophys Acta 437:313–321
O'Gara F, Treacy P, O'Sullivan D, O'Sullivan M, Higgins P (1986) Biological control of phytopathogens byPseudomonas sp.: genetic aspects of siderophore production and root colonisation. In: Swinburne TR (ed) Iron, siderophores and plant diseases. Plenum Press, New York, pp 331–339
O'Sullivan DJ, O'Gara F (1990) Iron regulation of ferric iron uptake in a fluorescent pseudomonad: cloning of a regulatory gene. Mol Plant Microbe Interact 3:86–93
O'Sullivan DJ, O'Gara F (1991) Regulation of iron assimilation: nucleotide sequence analysis of an iron-regulated promoter from a fluorescent pseudomonad. Mol Gen Genet 228:1–8
O'Sullivan DJ, O'Gara F (1992) Traits of fluorescentPseudomonas sp. involved in suppression of plant root pathogens. Microbiol Rev 56:662–676
O'Sullivan DJ, Morris J, O'Gara F (1990) Identification of an additional ferric siderophore uptake gene clustered with receptor, biosynthesis, andfur-like regulatory genes in fluorescentPseudomonas sp. M114. Appl Environ Microbiol 56:2056–2064
O'Sullivan DJ, Dowling DN, de Lorenzo V, O'Gara F (1994)Escherichia coli ferric uptake regulator (Fur) can mediate regulation of a pseudomonad iron-regulated promoter. FEMS Microbiol Lett 117:327–332
Prince RW, Cox CD, Vasil ML (1993) Coordinate regulation of siderophore and exotoxin A production: molecular cloning and sequencing of thePseudomonas aeruginosa fur gene. J Bacteriol 175:2589–2598
Rombel IT, McMorran BJ, Lamont IL (1995) Identification of a DNA sequence motif required for expression of iron-regulated genes in pseudomonads. Mol Gen Genet 246:519–528
Scher FM, Baker R (1982) Effects ofPseudomonas putida and a synthetic iron chelator on induction of soil suppressiveness toFusarium wilt pathogens. Phytopathology 72:1567–1573
Sexton R, Gill PR Jr, Callanan MJ, O'Sullivan DJ, Dowling DN, O'Gara F (1995) Iron-responsive gene expression inPseudomonas fluorescens M114: cloning and characterisation of a transcription-activating factor PbrA. Mol Microbiol 15:297–306
Spaink HP, Okker RJH, Wiffelman CA, Pees E, Lugtenberg EJJ (1987) Promoters in the nodulation region of theRhizobium leguminosarum Sym plasmid pRL1J1. Plant Mol Biol 9:27–39
Van Hove B, Staudenmaier H, Braun V (1990) Novel two-component transmembrane transcription control: regulation of iron dicitrate transport inEscherichia coli K-12. J Bacteriol 172:6749–6758
Venturi V, Ottevanger C, Bracke M, Weisbeek P (1995) Iron regulation of siderophore biosynthesis and transport inPseudomonas putida WCS358: involvement of a transcriptional activator and of the Fur protein. Mol Microbiol 15:1081–1093
Wozniak DJ, Ohman DE (1994) Transcriptional analysis of thePseudomonas aeruginosa genesalgR, algB, andalgD reveals a heirarchy of alginate gene expression which is modulated byalgT. J Bacteriol 176:6007–6014
Xiao Y, Hutcheson SW (1994) A single promoter sequence recognized by a newly identified alternate sigma factor directs expression of pathogenicity and host range determinants inPseudomonas syringae. J Bacteriol 176:3089–3091
Xiao Y, Heu S, Yi J, Lu Y, Hutcheson SW (1994) Identification of a putative alternate sigma factor and characterisation of a multicomponent regulatory cascade controlling the expression ofPseudomonas syringae pv.syringae Pss61hrp andhrmA genes. J Bacteriol 176:1025–1036
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119
Zimmermann L, Hantke K, Braun V (1984) Exogenous induction of the iron dicitrate transport system ofEscherichia coli K-12. J Bacteriol 159:271–277
Author information
Authors and Affiliations
Additional information
Communicated by A. Kondorosi
Rights and permissions
About this article
Cite this article
Sexton, R., Gill, P.R., Dowling, D.N. et al. Transcriptional regulation of the iron-responsive sigma factor genepbrA . Molec. Gen. Genet. 250, 50–58 (1996). https://doi.org/10.1007/BF02191824
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02191824