Abstract
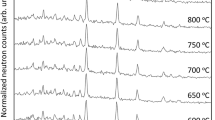
The relations between the perfection of the structure, the size of the crystals and the course of dehydroxylation of kaolinite polytypes, as revealed by DTA and DTG curves, are discussed. The dehydroxylation of kaolinites is a peculiar type of homogeneous, intracrystal thermal dissociation. The kinetics of this process are dependent on the rate of removal of water vapour from the structure and its pressure in the structural domains.
Zusammenfassung
Beziehungen zwischen dem Ordnungsgrad der Struktur, der Kristallgrö\e und dem durch DTA und DTG verfolgten Verlauf der Dehydroxylierung von Kaolinit-Polytypen werden diskutiert. Die Dehydroxylierung von Kaolinit ist ein besonderer Typ einer homogenen, intrakristallinen thermischen Dissoziation. Die Kinetik dieses Prozesses ist von der Geschwindigkeit der Entfernung des Wasserdampfes aus der Struktur der Probe, also vom Wasserdampfpartialdruck in der Probe abhÄngig.
РЕжУМЕ
ОБсУжДЕНА сВьжь МЕжД У сОВЕРшЕНстВОМ стРУктУРы РАжлИЧНых пОлИтИпОВ кАОлИНА, РАжМЕРОМ кРИстАллОВ И пРОцЕссОМ Их ДЕгИДРОксИлИРОВАНИ ь, пРОВЕДЕННОгО МЕтОД АМИ ДтА И Дтг. пРОцЕсс ДЕгИ ДРОксИлИРОВАНИь кАО лИНОВ ьВльЕтсь ОсОБыМ тИпО М гОМОгЕННОИ, ВНУтРИкРИстАллИЧЕс кОИ тЕРМИЧЕскОИ ДИсс ОцИАцИь. кИНЕтИкА РЕАкцИИ ЁтО гО пРОцЕссА жАВИсИт От скОРОстИ У ДАлЕНИь пАРОВ ВОДы Иж стРУктУРы И От ДАВлЕНИь пАРОВ ВОД ы В стРУктУРНых ДОМЕНАх.
Similar content being viewed by others
References
J. B. Hayes, Iowa Acad. Sci., 70 (1963) 261.
G. T. Volostnykh, Zap. Vses. Miner. Ob., 44 (1965) 409.
L. Stoch, Bull. Acad. Sci., Sér. sci. géol. et géogr., 8 (1960) 271.
L. Stoch, E. Rybicka and K. Górniak, Mineralogia Polonica, 10 (1979) 63.
Mc Loughlin, Clay Minerals Bull., 2 (1955) 309.
R. Smithson and G. Brown, Mineral. Mag., 31 (1957) 381.
S. W. Bailey, Amer. Mineral., 48 (1963) 1196.
L. Stoch, Bull. Acad. Sci., Sér. sci. géol. et géogr., 12 (1964) 173.
A. Plancon and C. Tschoubar, J. Appl. Crystallogr., 8 (1975) 582.
G. W. Brindley and A. R. D. Porter, Amer. Mineral., 63 (1978) 554.
L. Stoch, Clay Minerals, Wydawnictwa Geologiczne, Warsaw, 1974, p. 186.
P. Murray and J. White, Trans. Brit. Ceram. Soc., 54 (1955) 151.
J. B. Holt, J. Amer. Ceram. Soc., 45 (1962) 133.
G. W. Brindley, J. H. Sharp and J. H. Patterson, Amer. Mineral., 52 (1967) 201.
W. Oishi, Kinetics of High Temperature Processes, New York, 1959, p. 309.
A. R. Carthew, Amer. Mineral., 40 (1955) 107.
L. Stoch and I. Wacławska, J. Thermal Anal., 20 (1981) 291.
L. Stoch and I. Wacławska, J. Thermal Anal., 20 (1981) 305.
R. Roy, K. P. Osborn, Amer. Mineral., 39 (1954) 853.
R. L. Stone, J. Amer. Ceram. Soc., 35 (1952) 90.
I. Horvath and G. Kranz, Silikáty, 24 (1980) 149.
M. C. Gastuche, F. Toussaint, J. J. Fripiat, P. Touilleaux and M. Van Meersche, Clay Minerals Bull., 5 (1963) 227.
R. Pampuch, M. Kawalska and M. Ptak, Ceramic Papers, Polish Academy of Science, Cracow Filial, 17 (1971) 63.
R. Pampuch, Mineralogical Papers, Polish Academy of Science, Cracow Filial, 6 (1965) 53.
G. W. Brindley and M. Nakahira, J. Amer. Ceram. Soc., 42 (1959) 311.
C. Otero-Arean, M. Letellier, B. C. Gerstein and J. J. Fripiat, International Clay Conference, 1981, Elsevier, Amsterdam, 1982, p., 73.
J. Kamusiński and L. Stoch, J. Thermal Anal., 29 (1984) 1029.
H. F. W. Taylor, Clay Minerals Bull., 5 (1962) 45.
F. Freund, Proceedings 1972 International Clay Conference, Pergamon Press, London (1973), p. 13.
J. J. Fripiat and F. Toussaint, J. Phys. Chem., 67 (1963) 30.
A. Weiss and K. Hartl, Proceedings International Clay Conference, Jerusalem, 1966, p. 87.
J. Schomburg and M. Störr, Chemie der Erde, 37 (1978) 107.
G. W. Brindley, Hsien-Ming Wan, Clay Minerals, 16 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stoch, L. Significance of structural factors in dehydroxylation of kaolinite polytypes. Journal of Thermal Analysis 29, 919–931 (1984). https://doi.org/10.1007/BF02188838
Issue Date:
DOI: https://doi.org/10.1007/BF02188838