Abstract
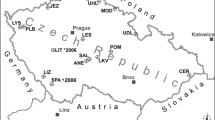
The Experimental Watershed Liming Study (EWLS) was initiated to evaluate the application of CaCO3 to a forested watershed in an effort to mitigate the acidification of surface water. The objective of the EWLS was to assess the response of the Woods Lake watershed to an experimental addition of CaCO3. During October 1989, 6.89 Mg CaCO3/ha was applied by helicopter to two subcatchments comprising about 50% (102.5 ha) of the watershed area. The EWLS involved individual investigations of the response of soil and soil water chemistry, forest and wetland vegetation, soil microbial processes, wetland, stream and lake chemistry, and phytoplankton and fish to the CaCO3 treatment. In addition, the Integrated Lake/Watershed Acidification (ILWAS) model was applied to the site to evaluate model performance and duration of the treatment. The results of these studies are detailed in this volume. The purposes of this introduction and synthesis paper are to: 1) present the overall design of the EWLS, 2) discuss the linkages between the individual studies that comprise the EWLS, and 3) summarize the response of the lakewater chemistry to watershed addition of CaCO3 and compare these results to previous studies of direct lake addition. An analysis of lake chemistry revealed the watershed treatment resulted in a gradual change in pH, acid neutralizing capacity (ANC) and Ca2+ in the water column. This pattern was in contrast to direct lake additions of CaCO3 which were characterized by abrupt changes following base addition and subsequent rapid reacidification. Over the three-year study period, the supply of ANC to drainage waters was largely derived from dissolution of CaCO3 in wetlands. Relatively little dissolution of CaCO3 occurred in freely draining upland soils. The watershed treatment had only minor effects on forest vegetation. The watershed treatment eliminated the episodic acidification of streamwater and the near-shore region of the lake during snowmelt, a phenomenon that occurred during direct lake treatments. Positive ANC water in the near-shore area may improve chemical conditions for fish reproduction, and allow for the development of a viable fish population. The watershed CaCO3 treatment also decreased the transport of Al from the watershed to the lake, and increased the concentrations of dissolved organic carbon (DOC) and dissolved silica (H4SiO4) in stream and lakewater. The watershed treatment appeared to enhance soil nitrification, increasing concentrations of NO3 − in soilwater and surface waters. However, the acidity associated with this NO3 − release was small compared to the increase in ANC due to CaCO3 addition and did not alter the acid-base status of Woods Lake. Acid neutralizing capacity (ANC) budgets for 12-month periods before and after the watershed treatment showed that the lake shifted from a large source of ANC to a minor source due to retention of SO4 2−, NO3 −, Al and the elevated inputs of Ca2+ associated with the watershed CaCO3 application. In contrast to the direct lake treatments, Ca2+ inputs from the watershed application were largely transported from the lake.
Similar content being viewed by others
References
Aber, JD & Melillo SM (1991) Terrestrial Ecosystems. Saunders College Publishing, Philadelphia, PA
American Public Health Association (1985) Standard Methods for the Examination of Water and Wastewater. American Public Health Association, New York
April R & Newton RM (1985) Influence of geology on lake acidification in the ILWAS watersheds. Water Air Soil Pollut. 26: 373–386
Baker LA (1990) Regional estimates of atmospheric dry deposition. In: Charles DF (Ed) Acidic Deposition and Aquatic Ecosystems: Regional Case Studies (pp 645–653). Springer-Verlag, New York
Bengtsson B, Dickson W & Nyberg P (1980) Liming acid lakes in Sweden. Ambio 9: 34–36
Bennett PC, Siegel DI, Hill BM & Glaser PH (1991) Fate of silicate minerals in a peat bog. Geology 19: 328–331
Black CA, Evans DD, White JL, Ensminger LE & Clark FE (Eds) (1965) Methods of Soil Analysis: Part 2. Chemical and Microbiological Properties. American Society of Agronomy Monograph 9, Madison, WI
Blette VL & Newton RM (1996a) Effects of watershed liming on the soils of Woods Lake, New York. Biogeochemistry 32: 175–194 (this volume)
Blette VL & Newton RM (1996b) Application of the Integrated Lake-Watershed Acidification Study model to watershed liming at Woods Lake, New York. Biogeochemistry 32: 363–383 (this volume)
Brach AR & Raynal DJ (1992) Effects of liming on Oxalis acetosella and Lycopodium lucidulum in a northern hardwood forest. J. of Appl. Ecol. 29: 492–500
Bukaveckas P (1988) Effects of calcite treatment on primary producers in acidified Adirondack lakes — response of macrophyte communities. Lake Reser. Manage. 4: 107–113
Burns DA (1996) Effects of CaCO3 application to an Adirondack Lake watershed on outlet-stream chemistry. Biogeochemistry 32: 339–362 (this volume)
Butler TJ & Likens GE (1991) The impact of changing regional emissions on precipitation chemistry in the eastern United States. Atmos. Environ. 25A: 305–315
Cappo KA, Blume LJ, Raab GA, Bartz JK & Engels JL (1987) Analytical Methods Manual for the Direct/Delayed Response Project Soil Survey. United States Environmental Protection Agency, Environmental Monitoring Systems Laboratory, Office of Research and Development, Las Vegas, NV. EPA 600/8-87/020
Charles, DF (Ed) (1991) Acidic Deposition and Aquatic Ecosystems: Regional Case Studies. Springer-Verlag, New York
Cirmo CP & Driscoll CT (1996) The impacts of a watershed CaCO3 treatment on stream and wetland biogeochemistry in the Adirondack Mountains. Biogeochemistry 32: 265–297 (this volume)
Cronan CS, Conlan JC & Skibinski S (1987) Forest vegetation in relation to surface water chemistry in the North Branch of the Moose River, Adirondack Park, NY. Biogeochemistry 3: 121–128
Davis JE (1988) Simulated response of an acidic Adirondack lake watershed to various liming mitigation strategies. Water Resour. Res. 24: 525
Dillon PJ & Scheider WA (1984) Modelling the reacidification rates of neutralized lakes near Sudbury Ontario. In: Schnoor JL (Ed) Modelling of Total Acid Precipitation Inputs (pp 121–154) Butterworth Boston, MA
Dillon PJ, Reid RA & De Grosbois E (1987) The rate of acidification of aquatic ecosystems in Ontario, Canada. Nature 329, 45–48
Dohrmann (1984) Operator Manual. Automated Laboratory Total Carbon Analyzer. Zertex Corp., Santa Clara, CA
Driscoll CT (1984) A procedure for the fractionation of aqueous aluminum in dilute acidic waters. Int. J. Environ. Anal. Chem. 16: 267–284
Driscoll CT & van Dreason R (1993) Seasonal and long-term temporal patterns in the chemistry of Adirondack Lakes. Water Air Soil Pollut. 67: 319–344
Driscoll CT, Likens GE, Hedin LO, Eaton JS & Bormann FH (1989a) Changes in the chemistry of surface waters: 25-year results of the Hubbard Brook Experimental Forest, NH. Environ. Sci. Technol. 23: 137–142
Driscoll CT, Ayling WA, Fordham GF & Oliver LM (1989b) Chemical response of lakes treated with CaCO3 to reacidification. Can. J. Fish. Aquat. Sci. 46: 258–267
Driscoll CT, Fordham GF, Ayling WA & Oliver LM (1989c) Short-term changes in the chemistry of trace metals following calcium carbonate treatment. Can. J. Fish. Aquat. Sci. 46: 249–257
Driscoll CT, Lehtinen MD & Sullivan TJ (1994) Modeling the acid-base chemistry of organic solutes in Adirondack, NY, lakes. Water Resour. Res. 30: 297–306
Driscoll CT, Postek KM, Kretser W & Raynal DJ (1995) Long-term trends in the chemistry of precipitation and lake water in the Adirondack region of New York, USA. Water Air Soil Pollut. (in press)
Fordham GF & Driscoll CT (1989) Short term changes in the acid-base chemistry of two acidic lakes following calcium carbonate treatment. Can. J. Fish. Aquat. Sci. 46: 306–314
Geary R & Driscoll CT (1996) Forest soil solutions: acid/base chemistry and response to calcite treatment. Biogeochemistry 32: 195–220 (this volume)
Gherini SA, Mok L, Hudson RJM, Davis GF, Chen CW & Goldstein RA (1985) The ILWAS model: Formulation and application. Water Air Soil Pollut. 26: 425–459
Goldstein RA, Chen CW & Gherini SA (1985) Integrated Lake Watershed Acidification Study: Summary. Water Air Soil Pollut. 26: 327–337
Goldstein RA, Gherini SA, Chen CW, Mok L & Hudson RJM (1984) Integrated Lake Watershed Acidification Study (ILWAS): a mechanism ecosystem analysis. Trans. Royal Soc., London. B305: 409–425
Gran G (1952) Determination of the equivalence point in potentiometric titration. Int. Cong. Int. Chem. 77: 661–671
Gross MG (1971) Carbon Determination. In: Carver RJ (Ed) Procedures in Sedimentary Petrology,Wiley Interscience, New York
Gubala CP & Driscoll CT (1991) The chemical responses of acidic Woods Lake, NY to two different treatments with calcium carbonate. Water Air Soil Pollut. 59: 7–22
Gubala CP, Driscoll CT, Newton RM & Schofield CL (1991) Chemistry of a near-shore lake region during spring snowmelt. Environ. Sci. Technol. 25: 2024–2030
Hamon WR (1961) Estimating potential evapotranspiration. J. Hydraulics Div., ASCE: 107–120
Hedin LO, Likens GE & Bormann FH (1987) Decrease in precipitation acidity resulting from decreased SO4 2− concentration. Nature 325: 24–246
Hedin, LO, Granat L, Likens GE, Buishand TA, Galloway JN, Butler TJ & Rodhe H (1994) Steep declines in atmospheric base cations in regions of Europe and North America. Nature 367: 351–354
Henriksen A, Lien L, Rosseland, BO, Traaen TS & Sevaldrud IS (1989) Lake acidification in Norway: present and predicted fish status. Ambio 18: 314–321
Hiebert FK & Bennett PC (1992) Microbial control of silicate weathering in organic-rich ground water. Science 258: 278–281
Hultberg H & Andersson IB (1982) Liming of acidified lakes induced long-term changes. Water Air Soil Pollut. 18: 311–331
Husar RB, Sullivan TJ & Charles DB (1991) Historical trends in atmospheric sulfur deposition and methods for assessing long-term trends in surface water chemistry In: Charles DB (Ed) Acidic Deposition and Aquatic Ecosystems: Regional Case Studies, Springer-Verlag, New York
Jeffries DS, Wales DL, Kelso, JRM & Linthurst RA (1986) Regional chemical characteristics of lakes in North America. Part 1 — Eastern Canada. Water Air Soil Pollut. 31: 551–567
Keeney DR & Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL, Miller RH & Keeney DR (Eds) Methods of Soil Analysis: Part 2. 2nd Edition. ASA-ASSA, Inc. Madison, WI
Kretser W, Gallagher J & Nicolette J (1989) Adirondack Lake Study, 1984–1987: An Evaluation fo Fish Communities and Water Chemistry. Adirondack Lakes Survey Corporation, Ray Brook, NY
Mackun IR, Leopold DJ & Raynal DJ (1994) Short-term responses of wetland vegetation after liming of an Adirondack watershed. Ecol. Appl. 4: 535–543
McAvoy DW, Santore, RC, Shosa JD & Driscoll CT (1992) Comparison between pyrocatechol violet and 8-hydroxyquinoline procedures for determining aluminum fractions. Soil Sci. Soc. Am. J. 56: 449–455
Morgan MD (1990) Streams in the New Jersey Pinelands directly reflect changes in atmospheric deposition chemistry. J. Environ. Qual. 19: 296–302
New York State Atmospheric Deposition Monitoring Network (1987–1991) Wet Deposition, Nick"s Lake. New York State Department of Environmental Conservation, Division of Air Resources, Albany, NY
Newton RM, Bums DA, Blette VL & Driscoll CT (1996) Effect of whole catchment liming on the episodic acidification of two Adirondack streams. Biogeochemistry 32: 299–322 (this volume)
Olem H (1990) Liming acidic surface waters: Report 15. In: Irving PM (Ed) Acidic Deposition: State of Science and TechnologyNational Acid Precipitation Assessment Program, Washington, DC
Orion (1976) Instruction Manual Fluoride Electrodes. Orion Research Inc., Cambridge, MA
Porcella D (1989) Mitigation of acidic conditions in lakes: an overview of an ecosystem perturbation experiment. Can. J. Fish. Aquat. Sci. 46: 246–248
Rossell IM, Leopold DJ & Raynal DJ (1995) Competition among shoreline plants: responses to watershed liming. Env. Exp. Botany (in press)
Rossell IM, Raynal DJ & Leopold DJ (1994) The effects of watershed liming on the tissue chemistry of three co-occurring poor fen species. Can. J. Bot. 72: 1825–1834
Schecher WD & Driscoll CT (1995) ALCHEMI: A chemical equilibrium model to assess the acid-base chemistry and speciation of aluminum in dilute solutions. In: Loeppert R, Schwab AP & Goldberg S (Eds) Chemical Equilibrium and Reaction Models (pp 325–356) Soil Sci. Soc. America, Madison, WI
Schofield CL (1993) Habitat suitability for brook trout (Salvelinus fontinalis) reproduction in Adirondack lakes. Water Resour. Res. 77: 875–879
Schofield CL & Keleher C (1996) Comparison of brook trout reproductive success and recruitment in an acidic Adirondack lake following whole lake liming and watershed liming. Biogeochemistry 32: 323–338 (this volume)
Shepard JP, Mitchell MJ, Scott T, Zang Y & Raynal DJ (1989) Measurements of wet and dry deposition in a northern hardwood forest. Water Air Soil Pollut. 48: 225–238
Simmons JA (1993) Lime Effects on Carbon and Nitrogen Dynamics of a Northern Hardwood Forest Floor. Ph.D. thesis. Cornell University, Ithaca, NY
Simmons JA, Yavitt JB & Fahey TJ (1996) Response of forest floor nitrogen dynamics to liming. Biogeochemistry 32: 221–244 (this volume)
Slavin W (1968) Atomic Absorption Spectroscopy. Wiley Interscience, New York
Smallidge, PJ & Leopold DJ (1994) Forest community composition and juvenile red spruce (Picea rubens) age-structure and growth patterns in an Adirondack watershed. Bull. Torry Bot. Club 121: 345–356
Smallidge PJ & Leopold DJ (1995) Watershed liming and pit and mound topography effects on seed banks in the Adirondacks, New York, USA. For. Ecol. Mgmt. 72: 273–286
Smallidge PJ, Brach AR & Mackun IR (1993) Effects of watershed liming on terrestrial ecosystem processes. Environ. Rev. 1: 157–171
Staubitz WW & Zariello PJ (1989) Hydrology of two headwater lakes in the Adirondack Mountains of New York. Can. J. Fish. Aquatic Sci. 46: 268–276
Stumm W & Morgan JJ (1981) Aquatic Chemistry. Johns Wiley & Sons, New York
Tabatabai MA & Dick WA (1983) Simlutaneouos determination of nitrate, chloride, sulfate, and phosphate in natural waters by ion chromatography. J. Environ. Qual. 12: 209–213
Weatherbum MW (1967) Phenylhypochlorite reaction for determination of ammonia. Analytica Chemica 39: 971–973
Wright RF (1985) Liming and reacidification of Hovvatan a chronically acidified lake in southernmost Norway. Can. J. Fish. Aquat. Sci. 42: 1103–1113
Yavitt JB & Fahey TJ (1996) Peatland porewater chemical responses to CaCO3 applications in wetlands next to Woods Lake, New York. Biogeochemistry 32: 245–263 (this volume)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Driscoll, C.T., Cirmo, C.P., Fahey, T.J. et al. The experimental watershed liming study: Comparison of lake and watershed neutralization strategies. Biogeochemistry 32, 143–174 (1996). https://doi.org/10.1007/BF02187137
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02187137