Abstract
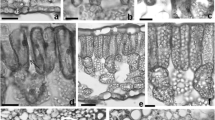
Scots pine (Pinus sylvestris L.) seedlings were fumigated with 1.2–1.5 x ambient ozone over 2 seasons in an open-air experiment. Fumigation started in the early spring and continued into late autumn during both years. Needle and root cell structures were analyzed in the summer, autumn and early winter following the second fumigation period. Under the light microscope an increase in the intercellular space and disintegrating cells in the mesophyll tissue near the stomata and stomatal cavities were observed in the ozone-exposed needles. Darkening of chloroplast stroma, increased plastoglobulus size and decreased chloroplast size were characteristic ultrastructural changes associated with ozone exposure. In addition, less dense grouping of the chloroplasts in the needles of elevated ozone-exposed seedlings as compared to the controls (background ozone) was observed in the early winter. Fewer starch grains and an increased accumulation of tannin-like substances were detected in both mycorrhizal and uninfected roots of ozone-exposed seedlings as compared to the control seedlings. For the first time, we were able to show that the ozone-induced darkening of needle chloroplast stroma is a reversible symptom. An increased frequency of frost injury symptoms indicated that the winter hardening process was disturbed in the needles of ozone-treated seedlings.
Similar content being viewed by others
References
Allen SE (1989) Chemical analysis of ecological material. Blackwell Scientific, Oxford
Anttonen S (1992) Changes in lipids ofPinus sylvestris needles exposed to industrial air pollution. Ann Bot Fenn 29:89–99
Anttonen S, Kärenlampi L (1995) Fatty acid, starch and biomass of Scots pine needles and roots in open-air ozone exposure. Trees (in press)
Anttonen S, Herranen J, Peura P, Kärenlampi L (1995) Fatty acids and ultrastructure of ozone-exposed Aleppo pine (Pinus halepensis Mill.) needles. Environ Pollut 87:235–242
Back J (1994) Effects of acidic precipitation on Scots pine and Norway spruce needles and their low temperature tolerance. Acta Universitatis Ouluensis Series A. Scientiae Rerum Naturalium 254 PhD thesis
Barnes JD, Davison AW (1988) The influence of ozone on the winter hardiness of Norway spruce [Picea abies (L.) Karst.]. New Phytol 108:159–166
Brown KA, Roberts TM, Blank LW (1988) Interaction between ozone and cold sensitivity in Norway spruce: a factor contributing to the forest decline in central Europe? New Phytol 105:149–155
Carlsson A, Hellgren LI, Sellden G, Sandelius A-S (1994) Effect of moderately enhanced levels of ozone on the acyl lipid composition of leaves of garden pea (Pisum sativum) Physiol Plant 91:754–762
Castillo FJ, Heath RL (1990) Ca+ transport in membrane vesicles from pinto bean leaves and its alteration after ozone exposure. Plant Physiol 94:788–795
Chappelka AH, Chevone BI (1992) Tree responses to ozone. In: Lefohn A (ed) Surface level ozone exposures and their effects on vegetation. Lewis, Chelsea, Mich., pp 271–324
Chappelka AH, Freer-Smith PH (1995) Prodisposition of trees by air pollutants to low temperatures and moisture stress. Environ Pollut 87:105–117
Cooke DT, Burden RS (1990) Lipid modulation of plasma membrane-bound ATPase. Physiol Plant 78:153–159
Darrall NM (1989) The effect of air pollutants on physiological processes in plants. Plant Cell Environ 12:1–30
DeHayes DH, Thornton FC, Waite CE, Ingle MA (1991) Ambient cloud deposition reduces cold tolerance of red spruce seedlings. Can J For Res 21:1292–1295
Douglas KT (1987) Mechanisms of action of glutathione-dependent enzymes. In: Meister A (ed) Advances in enzymology. John Wiley, New York, pp 103–167
Ebel B, Rosenkrantz J, Schiffens A, Lutz C (1990) Cytological observations on spruce needles after prolonged treatment with ozone and acid mist. Environ Pollut 64:323–335
Evans LS, Miller PR (1972) Ozone damage to ponderosa pine: a histological and histochemical appraisal. Am J Bot 59:297–304
Fink S (1988) Histological and cytological changes caused by air pollutants and other abiotic factors. In: Schulte-Hostede N, Darral LW, Wellburn AR (eds) Air pollution and plant metabolism. Elsevier Applied Sciences, London, pp 36–54
Fincher J, Alscher RG (1992) The effect of long-term ozone exposure on injury in seedlings of red spruce (Picearubens Sarg.). New Phytol 120:49–59
Fincher J, Cumming JR, Alscher RG, Rubin G, Weinstein L (1989) Long-term ozone exposure affects winter hardiness of red spruce (Picea rubens Sarg.). New Phytol 113:85–96
Freeman BA, Platt-Aloia K, Mudd JB, Thomson WW (1977) Ultrastructural and lipid changes associated with the aging of citrus leaves. Protoplasma 93:220–233
Greitner CS, Pell EJ, Winner WE (1994) Analysis of aspen foliage exposed to multiple stresses: ozone, nitrogen deficiency and drought. New Phytol 127:579–589
Guiamet JJ, Giannibelli MC (1994) Inhibition of the degradation of chloroplast membranes during senescence in nuclear ‘stay green’ mutants of soybean. Physiol Plant 91:395–402
Halliwell B, Gutteridge JMC (1989) Free radicals in biology and medicine. Clarendon, Oxford
Hayes T, Lindgren FT, Gofman JW (1963) A quantitative determination of the osmium tetroxide-lipoprotein interaction. J Cell Biol 19:251–255
Heath RL (1987) The biochemistry of ozone attack on the plasma membrane of plant cells In: Saunders JA, Kosak-Channing L, Conn EE (eds) Recent advances in phytochemistry: phytochemical effects of environmental compounds, vol 21. Plenum Press, New York, pp 29–54
Hecht-Buchholz C, Jorns CA, Keil P (1987) Effect of excess aluminum and manganese on Norway spruce seedlings as related to magnesium nutrition. J Plant Nutr 10:1103–1110
Hess JL (1994) Free radical scavenging. In: Alscher RG, Wellburn AR (eds) Plant responses to the gaseous environment. Chapman and Hall, London, pp 99–120
Holopainen JK, Holopainen T (1988) Cellular responses of Scots pine (Pinus sylvestris L.) seedlings to simulated summer frost. Eur J For Path 18:207–216
Holopainen T (1989) Ecological and ultrastructural responses of Scots pine mycorrhizas to industrial pollution. Agric Ecosystems Environ 28:185–189
Holopainen T, Heinonen-Tanski H (1993). Effects of different nitrogen sources on the growth of Scots pine seedlings and the ultrastructure and development of their mycorrhizae. Can J For Res 23:262–272
Holopainen T, Nygren P (1989) Effect of potassium deficiency and simulated acid rain, alone or in combination, on the ultrastructure of Scots pine needles. Can J For Res 19:1402–1411
Holopainen T, Anttonen S, Palomäki V, Kainulainen P, Holopainen JK (1995) Needle ultrastructure and starch content in Scots pine and Norway spruce after ozone fumigation. Can J Bot (in press)
Kärenlampi LK, Friendland AJ (1988) Cytopathological and external observations of red spruce (Picea rubens Sarg.) needles damaged in winter in Vermont. Silva Fenn 22:249–255
Kärenlampi L, Houpis JLJ (1986) Structural conditions of mesophyll cells ofPinus ponderosa var. scopulorum after SO2 fumigation. Can J For Res 16:1381–1385
Krupa SV, Manning WJ (1988) Atmospheric ozone: formation and effects on vegetation. Environ Pollut 50:101–137
Lucas PW, Cottam DA, Sheppard LJ, Francis BJ (1988) Growth responses and delayed winter hardening in Sitka spruce following summer exposure to ozone. New Phytol 108:495–504
Martin B, Öquist G (1979) Seasonal and experimentally induced changes in the ultrastructure of chloroplasts ofPinus silvestris. Physiol Plant 46:42–49
McQuattie CJ, Schier GA (1992) Effect of ozone and aluminum on pitch pine (Pinus rigida) seedlings: anatomy of mycorrhizae. Can J For Res 22: 1901–1916
McQuattie CJ, Schier GA (1993) Effect of ozone and aluminum on pitch pine (Pinus rigida) seedlings: needle ultrastructure. Can J For Res 23:1375–1387
Meyberg M, Lockhausen J, Kristen U (1988) Ultrastructural changes in mesophyll cells of spruce needles from a declining forest in Northern Germany. Eur J For Path 18:169–175
Miyake H, Matsumura H, Fujinuma Y, Totsuka T (1989) Effects of low concentration of ozone on the fine strcuture of radish leaves. New Phytol 111:187–195
Ojanperä K, Sutinen S, Pleijel H, Sellden G (1992) Exposure of spring wheat,Triticum aestivum, L. cv. Drabant, to different concentrations of ozone in open-top chambers: effects on ultrastructure of flag leaf cells. New Phytol 120:39–48
Palomäki V, Holopainen T (1995) Effects of nitrogen deficiency and recovery fertilization on ultrastructure, growth and mineral concentrations of Scots pine needles. Can J For Res 24: 2459–2468
Pell EJ (1987) Ozone toxicity — is there more than one mechanism of action? In: Hutchinson TC, Meema KM (eds) Effects of atmospheric pollutants on forests, wetlands and agricultural ecosystems. NATO ASI Ser, vol G16. Springer, Berlin Heidelberg New York, pp 229–240
Pell EJ, Eckardt NA, Enyedi AJ (1992) Timing of ozone stress and resulting status of ribulose biphosphate carboxylase/oxygenase and associated net photosynthesis. New Phytol 120:397–405
Pell EJ, Eckardt NA, Glick RE (1994) Biochemical and molecular basis for impairment of photosynthetical potential. Photosynt Res 39:453–462
Raitio H (1990) The foliar chemical composition of young pines (Pinus sylvestris L.) with or without decline. In: Kauppi P, Kenttämies K, Anttila P (eds) Acidification in Finland. Springer, Berlin Heidelberg New York, pp 699–713
Reinikainen J, Huttunen S (1989) The level of injury and needle ultrastructure of acid rain-irrigated pine and spruce seedlings after low temperature treatment. New Phytol 112:29–39
Runeckles VC, Chevone BI (1992) Crop responses to ozone. In: Lefohn A (ed) Surface level ozone exposures and their effects on vegetation. Lewis, Chelsea, Mich., pp 189–270
Sakaki T, Kondo N, Yamada M (1990) Pathway for the synthesis of triacylglycerols from monogalactocyldiacylglycerols in ozone-fumigated spinach leaves. Plant Physiol 94:773–780
Sakaki T, Tanaka K, Yamada M (1994) General metabolic changes in leaf lipids in response to ozone. Plant Cell Physiol 35:53–62
SAS Institute (1988) SAS Procedures guide, release 6.03 edition. Cary, N.C.
Sasek TW, Richardson CJ, Fendick EA, Bevington SR, Kress LW (1991) Carry over effects of acid rain and ozone on the physiology of multiple flushes of loblolly pine seedlings. For Sci 4:1078–1098
Schiffgens-Gruber A, Lütz C (1992) Ultrastructure of mesophyll cell chloroplasts of spruce needles exposed to O3, SO2 and NO2 alone and in combination. Env Exp Bot 32:243–254
Soikkeli S (1978) Seasonal changes in mesophyll ultrastructure of needles of Norway spruce (Picea abies). Can J Bot 56:1932–1940
Soikkeli S (1980) Ultrastructure of the mesophyll in Scots pine and Norway spruce: seasonal variation and molarity of the fixative buffer. Protoplasma 103:241–252
Sutinen S (1987) Ultrastructure of mesophyll cells of spruce needles exposed to ozone alone and together with SO2. Eur J For Path 17:362–368
Sutinen S, Skärby L, Wallin G and Sellden G (1990) Long-term exposure of Norway sprucePicea abies (L.) Karst, to ozone in open-top chambers II. Effects on the ultrastructure of the needles. New Phytol 115:345–355
Sutton R, Ting IP (1977) Evidence for repair of ozone induced membrane injury: alteration in sugar uptake Atmos Environ 11:273–275
Tevini M, Steinmüller D (1985) Composition and function of plastoglobuli. II Lipid composition of leaves and plastoglobuli during beech leaf senescence. Planta 163:91–96
Waite CE, DeHayes DH, Rebbeck J, Schier GA, Johnson AH (1994) The influence of elevated ozone on freezing tolerance of red spruce seedlings. New Phytol 126:327–335
Walles B, Nyman B, Allen T (1973) On the ultrastructure of needles ofPinus sylvestris L. Stud For Suec 106:1–26
Wallin G, Skärby L (1992) The influence of ozone on the stomatal and non-stomatal limitation of photosynthesis in Norway spruce,Picea abies (L.) Karst, exposed to soil moisture deficit. Trees 6:128–136
Wellburn FAM, Wellburn AR (1994) Atmospheric ozone affects carbohydrate allocation and winter hardiness ofPinus halepensis (Mill.). J Exp Bot 274:607–614
Wulff A, Hänninen O, Tuomainen A, Kärenlampi L (1992) A method for open-air exposure of palnts to ozone. Ann Bot Fenn 29:253–262
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anttonen, S., Kärenlampi, L. Slightly elevated ozone exposure causes cell structural changes in needles and roots of Scots pine. Trees 10, 207–217 (1996). https://doi.org/10.1007/BF02185671
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02185671