Abstract
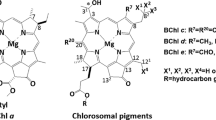
Artificial aggregates of bacteriochlorophyllc (BChlc) were formed in an aqueous medium in the presence of a lipid, monogalactosyl diglyceride (MGDG), and the optical properties of those aggregates were studied by absorption and circular dichroism (CD) mainly. Four BChlc homologs, ([E,E]BChlc F, [P,E]BChlc F, [E,M]BChlc F and [I,E]BChlc F), were isolated from the green photosynthetic bacteriumChlorobium limicola strain 6230. Above 0.0004%, MGDG induced a red-shift of the absorption maxima of BChlc aggregates. At 0.003% MGDG BChlc aggregates showed absorption maxima in the range of 724 to 745 (±3) nm with a shift of 12 to 24 (±3) nm depending on the homolog species. Four kinds of BChlc-MGDG aggregates showed characteristic CD spectra. [E,M]BChlc F gave rise to a CD spectrum similar to that of chlorosomes, while the other three gave spectra of opposite sign. These aggregates are sensitive to 1-hexanol treatment; in a saturating amount (0.85%) of 1-hexanol, all the homologs gave a monomer-like absorption spectrum peaking at 670nm. At an intermediate concentration (0.5%), [E,M]BChlc F showed an enhanced CD intensity, as observed in native chlorosomes. Resonance Raman spectra of the monomer-like BChlc samples indicated that the keto vibrational band at ca. 1640 cm−1 was considerably weakened by the 0.85% 1-hexanol treatment, however the 1680 cm−1 band characteristic of a free keto group did not appear. These results indicate that the artificial aggregates formed by purified BChlc homologs and MGDG are good models for studying chlorosomes structure.
Similar content being viewed by others
Abbreviations
- BChl:
-
bacteriochlorophyll
- CD:
-
circular dichroism
- MGDG:
-
monogalactosyl diglyceride
- [E,E]BChlc F :
-
8-ethyl-12-ethyl farnesyl BChlc
- [P,E]BChlc F :
-
8-propyl-12-ethyl farnesyl BChlc
- [E,M]BChlc F :
-
8-ethyl-12-methyl farnesyl BChlc
- [I,E]BChlc F :
-
8-isobutyl-12-ethyl farnesyl BChlc (This nomenclature is explained by Smith (1994))
References
Ballschmiter K and Katz JJ (1969) An infrared study of chlorophyllchlorophyll and chlorophyll-water interactions. J Am Chem Soc 91: 2661–2677
Blankenship RE, Brune DC, Freeman JM, King GH, McManus JD, Nozawa T, Trost T and Wittmershaus BP (1988) Energy trapping and electron transfer inChloroflexus aurantiacus. In: Olson JM, Ormerod JG, Amesz J, Stackebrandt E and Truper HG (eds) Green Photosynthetic Bacteria, pp 57–68. Plenum Press New York
Brune DC, Nozawa T and Blankenship RE (1987) Anntenna Organization in green photosynthetic bacteria. 1. Oligomeric bacteriochlorophyllc as a model for the 740 nm absorbing bacteriochlorophyllc inChloroflexus aurantiacus chlorosomes. Biochemistry 26: 8644–8652
Brune DC, Gerola PD and Olson JM (1990) Circular dichloism of green bacterial chlorosomes. Photosynth Res 24: 253–263
Causgrove TP, Cheng P, Brune DC and Blankenship RE (1993) Optical spectroscopy of a highly fluorescent aggregate of bacteriochlorophyllc. J Phys Chem 97: 5519–5524
Feick RG and Fuller RC (1984) Topology of the photosynthetic apparatus ofChloroflexus aurantiacus. Biochemistry 23: 3693–3700
Fujiwara M and Tasumi M (1986a) Resonance Raman and infrared studies on axial coordination to chlorophylla andb in vitro. J Phys Chem 90: 250–255
Fujiwara M and Tasumi M (1986b) Metal sensitive bands in the Raman and infrared spectra of intact and metal-substituted chlorophylla. J Phys Chem 90: 5646–5650
Griebenow K, Holtzwarth AR, vanMourik F and vaGrondelle R (1991) Pigment organization and energy transfer in green bacteria. 2. Circular and linear dichroism spectra of protein-containing and protein-free chlorosomes isolated fromChloroflexus aurantiacus strain Ok-70-fl. Biochim. Biophys. Acta 1058: 194–202
Hirota M, Moriyama T, Shimada K, Miller M, Olson JM and Matsuura K (1992a) High degree of organization of bacteriochlorophyllc in chlorosome-like aggregates spontaneously assembled in aqueous solution. Biochim Biophys Acta 1099: 272–274
Hirota M, Tsuji K, Shimada K and Matsuura K (1992b) Composition and organization of chlorosome-like bacteriochlorophyllc-lipid aggregates in aqueous solution. In: Murata N (ed) Research in Photosynthesis, Vol 1, pp 81–84. Kluwer Academic Publishers, Dordrecht, The Netherlands
Holzwarth AR, Griebenow K and Schaffner K (1990) A photosynthetic anntenna system which contains a protein-free chromophore aggregate. Z Naturforsch 45c: 203–296
Houssier C and Sauer K (1970) Circular dichroism and magnetic circular dichroism of the chlorophyll pigments. J Am Chem Soc 92: 779–791
Krasnovsky AA and Bystrova MI (1980) Self-assembly of chlorophyll aggregated structures. BioSystems 12: 181–194
Koyama Y, Umemoto Y, Akamatsu A, Uehara K and Tanaka M (1986) Raman spectra of chlorophyll forms. J Mol Struct 146: 273–287
Matsuura K and Olson JM (1990) Reversible conversion of aggregated bacteriochlorophyllc to monomeric form by 1-hexanol in chlorosomes fromChlorobium andChloroflexus. Biochim Biophys Acta 1019: 233–238
Matsuura K, Hirota M, Shimada K and Mimuro M (1993) Spectral forms and orientation of bacteriochlorophyllc anda in chlorosomes of the green photosynthetic bacteriumChloroflexus aurantiacus. Photochem Photobiol 57: 92–97
Miller M, Gillbro T and Olson JM (1993) Aqueous aggregates of bacteriochlorophyllc as a model for pigment organization in chlorosomes. Photochem Photobiol 57: 98–102
Nozawa T, Noguchi T and Tasumi M (1990) Resonance Raman Studies on the Structure of Bacteriochlorophyllc in chlorosomes fromChloroflexus aurantiacus. J Biochem 108: 737–740
Olson JM (1980) Chlorophyll organization in green photosynthetic bacteria. Biochim Biophys Acta 594: 33–51
Olson JM and Cox RP (1991) Monomers, dimers, and tetramers of 4-n-propyl-5-ethyl farnesyl bacteriochlorophyllc in dichloromethane and carbon tetrachloride. Photosynth Res 30: 35–43
Olson JM and Pedersen JP (1990) Bacteriochlorophyllc monomers, dimers and higher aggregates in dichloromethane, chloroform and carbon tetrachloride. Photosynth Res 25: 25–37
Risch N, Brockmann HJr and Gloe A (1979) Structure of new bacteriochlorophylls fromChloroflexus aurantiacus. Liebigs Ann Chem 1979: 408–418
Smith KM (1994) Nomenclature of the bacteriochlorophyllsc,d ande. Photosynth Res 41: 23–26 (special issue).
Smith KM, Craig GW, Kehres LA and Pfennig N (1983) Reversedphase high-performance liquid chromatography and structural assignments of the bacteriochlorophylls-c J Chromatogr 281: 209–223
Uehara K and Olson JM (1991) Aggregation of bacteriochlorophyllc homologs to dimers, tetramers, and polymers in water-saturated carbon tetrachloride. Photosynth Res 33: 251–257
Uehara K, Ozaki Y, Okada K and Olson JM (1991) FT-IR studies on the aggregation of Bacteriochlorophyllc fromChlorobium limicola. Chem Lett 1991: 909–912
Worcester DL, Michalski TJ and Katz JJ (1986) Small-angle neutron scattering studies of chlorophyll micelles: Models for bacterial anntenna chlorophyll. Proc Natl Acad Sci USA 83: 3791–3795
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Uehara, K., Mimuro, M., Ozaki, Y. et al. The formation and characterization of the in vitro polymeric aggregates of bacteriochlorophyllc homologs fromChlorobium limicola in aqueous suspension in the presence of monogalactosyl diglyceride. Photosynth Res 41, 235–243 (1994). https://doi.org/10.1007/BF02184164
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02184164