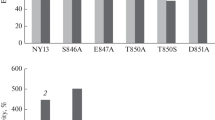
Abstract
The geneCAL1 (also known asCDC43) ofSaccharomyces cerevisiae encodes theβ subunit of geranylgeranyl transferase I (GGTase I), which modifies several small GTPases. Biochemical analyses of the mutant enzymes encoded bycall-1, andcdc43-2 tocdc43-7, expressed in bacteria, have shown that all of the mutant enzymes possess reduced activity, and that none shows temperature-sensitive enzymatic activities. Nonetheless, all of thecall/cdc43 mutants show temperature-sensitive growth phenotypes. Increase in soluble pools of the small GTPases was observed in the yeast mutant cells at the restrictive temperature in vivo, suggesting that the yeast prenylation pathway itself is temperature sensitive. Thecall-1 mutation, located most proximal to the C-terminus of the protein, differs from the othercdc43 mutations in several respects. An increase in soluble Rholp was observed in thecall-1 strain grown at the restrictive temperature. The temperature-sensitive phenotype ofcall-1 is most efficiently suppressed by overproduction of Rholp. Overproduction of the other essential target, Cdc42p, in contrast, is deleterious incall-1 cells, but not in othercdc43 mutants or the wild-type strains. Thecdc43-5 mutant cells accumulate Cdc42p in soluble pools andcdc43-5 is suppressed by overproduction of Cdc42p. Thus, several phenotypic differences are observed among thecall/cdc43 mutations, possibly due to alterations in substrate specificity caused by the mutations.
Similar content being viewed by others
References
Adams AEM, Johnson DI, Longnecker RM, Sloat BF, Pringle JR (1990)CDC42 andCDC43, two additional genes involved in budding and the establishment of cell polarity in the yeastSaccharomyces cerevisiae. J Cell Biol 111: 131–142
Adamson P, Marshall CJ, Hall A, Tilbrook PA (1992) Post-translational modifications of p21rho proteins. J Biol Chem 267: 20033–20038
Armstrong SA, Hannah VC, Goldstein JL, Brown MS (1995) CAAX geranylgeranyl transferase transfers farnesyl as efficiently as geranylgeranyl to RhoB. J Biol Chem 270: 7864–7868
Boguski MS, Murray AW, Powers S (1992) Novel repetitive sequence motifs in theα andβ subunits of prenyl-protein transferases and homology of theα subunit to theMAD2 gene product of yeast. New Biologist 4: 408–411
Caplin BE, Hettich LA, Marshall MS (1994) Substrate characterization of theSaccharomyces cerevisiae protein farnesyltransferase and type-I protein geranylgeranyltransferase. Biochim Biophys Acta 1205: 39–48
Chen W, Andres DA, Goldstein JL, Brown MS (1991a) Cloning and expression of a cDNA encoding theα subunit of rat p21ras protein farnesyltransferase. Proc Natl Acad Sci USA 88: 11368–11372
Chen W, Andres DA, Goldstein JL, Russell DW, Brown MS (1991b) cDNA cloning and expression of the peptide-bindingβ subunit of rat p21ras farnesyltransferase, the counterpart of yeastDPRI/RAMI. Cell 66: 327–334
Clarke S (1992) Protein isoprenylation and methylation at carboxylterminal cysteine residues. Annu Rev Biochem 61: 355–386
Diaz M, Sanchez Y, Bennett T, Sun CR, Godoy C, Tamanoi F, Duran A, Perez P (1993) TheSchizosaccharomyces pombe cwg2 − gene codes for the beta subunit of a geranylgeranyltransferase type I required for beta-glucan synthesis. EMBO J 12: 5245–5254
Dolence JM, Poulter CD (1995) A mechanism for posttranslational modification of proteins by yeast protein farnesyltransferase. Proc. Natl Acad Sci USA 92: 5008–5011
Finegold AA, Johnson DI, Farnsworth CC, Gelb MH, Judd SR, Glomset JA, Tamanoi F (1991) Geranylgeranyl: protein transferase ofSaccharomyces cerevisiae is specific for Cys-Xaa-Xaa-Leu motif proteins and requires theCDC43 gene product, but not theDPRI gene product. Proc Natl Acad Sci USA 88: 4448–4452
Fujimura K, Tanaka K, Nakano A, Toh-e A (1994) TheSaccharomyces cerevisiae MS14 gene encodes the yeast counterpart of component A of Rab geranylgeranyltransferase. J Biol Chem 269: 9205–9212
Goodman LE, Perou CM, Fujiyama A, Tamanoi F (1988) Structure and expression of yeastDPR1, a gene essential for the processing and intracellular localization of Ras proteins. Yeast 4: 271–281
He B, Chen P, Chen S, Vancura KL, Michaelis S, Powers S (1991)RAM2, an essential gene of yeast, andRAM1 encode the two polypeptide components of the farnesyltransferase that prenylates a-factor and Ras proteins. Proc Natl Acad Sci USA 88: 11373–11377
Ito H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153: 163–168
Johnson DI, Pringle JR (1990) Molecular characterization ofCDC42, aSaccharomyces cerevisiae gene involved in the development of cell polarity. J Cell Biol 111: 143–152
Johnson DI, O'Brien JM, Jacobs CW (1991) Isolation and sequence analysis ofCDC43, a gene involved in the control of cell polarity inSaccharomyces cerevisiae. Gene 98: 149–150
Kalman VK, Erdman RA, Maltese WA, Robishaw JD (1995) Regions outside of the CAAX motif influence the specificity of prenylation of G protein gamma subunits. J Biol Chem 270: 14835–14841
Kinsella BT, Erdman RA, Maltese WA (1991) Post-translational modification of Ha-ras p21 by farnesyl versus geranylgeranyl isoprenoids is determined by the COOH-terminal amino acid. Proc Natl Acad Sci USA 88: 8934–8938
Kohl NE, Diehl RE, Schaber MD, Rands E, Soderman DD, He B, Moores SL, Pompliano DL, Ferro-Novick S, Powers S, Thomas KA, Gibbs JB (1991) Structural homology among mammalian andSaccharomyces cerevisiae isoprenyl-protein transferases. J Biol Chem 266: 18884–18888
Madaule P, Axel R, Myers AM (1987) Characterization of two members of therho gene family from the yeastSaccharomyces cerevisiae. Proc Natl Acad Sci USA 84: 779–783
Mayer ML, Caplin BE, Marshall MS (1992)CDC43 andRAM2 encode the polypeptide subunits of a yeast type I protein geranylgeranyltransferase. J Biol Chem 267: 20589–20593
Mitsuzawa H, Esson K, Tamanoi F (1995) Mutant farnesyltransferase beta subunit ofSaccharomyces cerevisiae that can substitute for geranylgeranyltransferase type I beta subunit. Proc Natl Acad Sci USA 92: 1704–1708
Moomaw J, Casey PJ (1992) Mammalian protein geranylgeranyltransferase. Subunit composition and metal requirements. J Biol Chem 267: 17438–17443
Moores SL, Schaber MD, Mosser SD, Rands E, O'Hara MB, Garsky VM, Marshall MS, Pompliano DL, Gibbs JB (1991) Sequence dependence of protein isoprenylation. J Biol Chem 266: 14603–14610
Ohya Y, Ohsumi Y, Anraku Y (1984) Genetic study of the role of calcium ions in the cell division cycle ofSaccharomyces cerevisiae: a calcium-dependent mutant and its trifluoperazine-dependent pseudorevertants. Mol Gen Genet 193: 389–394
Ohya Y, Goebl M, Goodman LE, Petersen-Bjørn S, Friesen JD, Tamanoi F, Anraku Y (1991) YeastCAL1 is a structural and functional homologue to theDPR1 (RAM) gene involved in ras processing. J Biol Chem 266: 12356–12360
Ohya Y, Qadota H, Anraku Y, Pringle JR, Botstein D (1993) Suppression of yeast geranylgeranyl transferase I detect by alternative prenylation of two target GTPases, Rholp and Cdc42p. Mol Biol Cell 4: 1017–1025
Omer CA, Gibbs JB (1994) Protein prenylation in eukaryotic micro-organisms: genetics, biology and biochemistry. Mol Microbiol 11: 219–225
Orr-Weaver TL, Szostak JW, Rothstein RJ (1983) Genetic applications of yeast transformation with linear and gapped plasmids. Methods Enzymol 101: 228–245
Qudota H, Ishi I, Fujiyama A, Ohya Y, Anraku Y (1992)RHO gene products, putative small GTP-binding proteins, are important for activation of theCAL1/CDC43 gene product, a protein geranyl-geranyl-transferase inSaccharomyces cerevisiae. Yeast 8: 735–741
Qadota H, Anraku Y, Botstein D, Ohya Y (1994) Conditional lethality of a yeast strain expressing humanRHoA in place ofRHO1. Proc Natl Acad Sci USA 91: 9317–9321
Reiss Y, Goldstein JL, Seabra MC, Casey PJ, Brown MS (1990) Inhibition of purified p21ras farnesyl: protein transferase by Cys-AAX tetrapeptides. Cell 62: 81–88
Reiss Y, Seabra MC, Armstrong SA, Slaughter CA, Goldstein JL, Brown MS (1991a) Nonidentical subunits of p21H-ras farnesyltransferase. J Biol Chem 266: 10672–10677
Reiss Y, Stradley SJ, Gierasch LM, Brown MS, Goldstein JL (1991b) Sequence requirement for peptide recognition by rat brain p21ras protein farnesyltransferase. Proc Natl Acad Sci USA 88: 732–736
Rose MD, Winson (1990) Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Plainview, New York
Rossi G, Jiang Y, Newman AP, Ferro-Novick S (1991) Dependence of Ypt1 and Sec4 membrane attachment on Bet2. Nature 351: 158–161
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual (2nd edn). Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Schafer WR, Rine J (1992) Protein prenylation: genes, enzymes, targets and functions. Annu Rev Genet 26: 209–237
Schiest RH, Gietz RD (1989) High efficiency transformation of intact yeast cells using single-stranded nucleic acids as a carrier. Curr Genet 16: 339–346
Seabra MC, Reiss Y, Casey PJ, Brown MS, Goldstein JL (1991) Protein farnesyltransferase and geranylgeranyltransferase share a commonα subunit. Cell 65: 429–434
Seabra MC, Brown MS, Slaughter CA, Südhof TC, Goldstein JL (1992) Purification of component A of Rab geranylgeranyl transferase: possible identity with the choroideremia gene product. Cell 70: 1049–1057
Seabra MC, Brown MS, Goldstein JL (1993) Retinal degeneration in choroideremia: deficiency of Rab geranylgeranyl transferase. Science 259: 377–381
Sikorski RS, Hieter P (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA inSaccharomyces cerevisiae. Genetics 122: 19–27
Trueblood CE, Ohya Y, Rine J (1993) Genetic evidence for in vivo cross-specificity of the CaaX-box protein prenyltransferases farnesyltransferase and geranylgeranyltransferase-I inSaccharomyces cerevisiae. Mol Cell Biol 13: 4260–4275
Vieira J, Messing J (1987) Production of single-stranded plasmid DNA. Methods Enzymol 153: 3–11
Yokoyama K, Gelb MH (1993) Purification of a mammalian protein geranylgeranyltransferase. Formation and catalytic properties of an enzyme-geranylgeranyl pyrophosphate complex. J Biol Chem 268: 4055–4060
Yokoyama K, Goodwin GW, Ghomashchi F, Glomset JA, Gelb MH (1991) A protein geranylgeranyltransferase from bovine brain: implications for protein prenylation specificity. Proc Natl Acad Sci USA 88: 5302–5306
Yokoyama K, McGeady P, Gelb MH (1995) Mammalian protein geranylgeranyltransferase-I: substrate specificity, kinetic mechanism, metal requirements, and affinity labeling. Biochemistry 34: 1344–1354.
Zhang FL, Diehl RE, Kohl NE, Gibbs JB, Giros B, Casey PJ, Omer CA (1994) cDNA cloning and expression of rat and human protein geranylgeranyltransferase type-I. J Biol Chem 269: 3175–3180
Ziman M, Preuss D, Mulholland J, O'Brien JM, Protstein D, Johnson DI (1993) Subcellular localization of Cdc42p, aSaccharomyces cerevisiae GTP-binding protein involved in the control of cell polarity. Mol Biol Cell 4: 1307–1316
Author information
Authors and Affiliations
Additional information
Communicated by C. P. Hollenberg
Rights and permissions
About this article
Cite this article
Ohya, Y., Caplin, B.E., Qadota, H. et al. Mutational analysis of theβ-subunit of yeast geranylgeranyl transferase I. Molec. Gen. Genet. 252, 1–10 (1996). https://doi.org/10.1007/BF02173199
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02173199